Part:BBa_K3211605
Purification of Gp17 with a Cleavable N-terminal Twin-Strep Tag
Our cleavable Twin-Strep-tag collection is the perfect toolbox for the high-yield purification of any protein of interest. The Twin-Strep tag (BBa_K3211600), formed by the fusion of two StrepII tags (BBa_T2013), significantly increases the affinity towards the StrepTactinXT (an engineered form of streptavidin, IBA) binding resin and therefore improves the protein yield. However, the large size of this tag increases the interference with the folding and functioning of the purified protein. We have overcome this problem by introducing a factor Xa cleavage site (BBa_K3211601) between the tag and the coding sequence that allows the removal of the tag. Usually, one of the two termini of a folded protein is more accessible to the resin. This cleavable tag can be fused to either terminus of the protein enabling its optimal positioning (N-terminal cleavable tag: BBa_K3211603, C-terminal cleavable tag: BBa_K3211604). The modular assembly of any combination of tag, cleavage site and location provides a flexible toolbox for purification. We successfully purified the T7 phage tail fiber protein Gp17 (BBa_K3211602) and were able to cleave the tag.
Purification of Gp17 with the Cleavable N-terminal Twin-Strep Tag
All products for protein purification were received from IBA Life Sciences. Strep-TactinXT spin columns were used to purify the Gp17 protein. Note, that those columns are designed for rapid construct screening and not for high yield purification of the protein of interest (https://www.iba-lifesciences.com/strep-tactin-xt-system-spin-columns.html). The composite part was cloned into a vector that allows for the inducible overexpression of the protein. A detailed description of the cloning and purification procedure can be downloaded here.
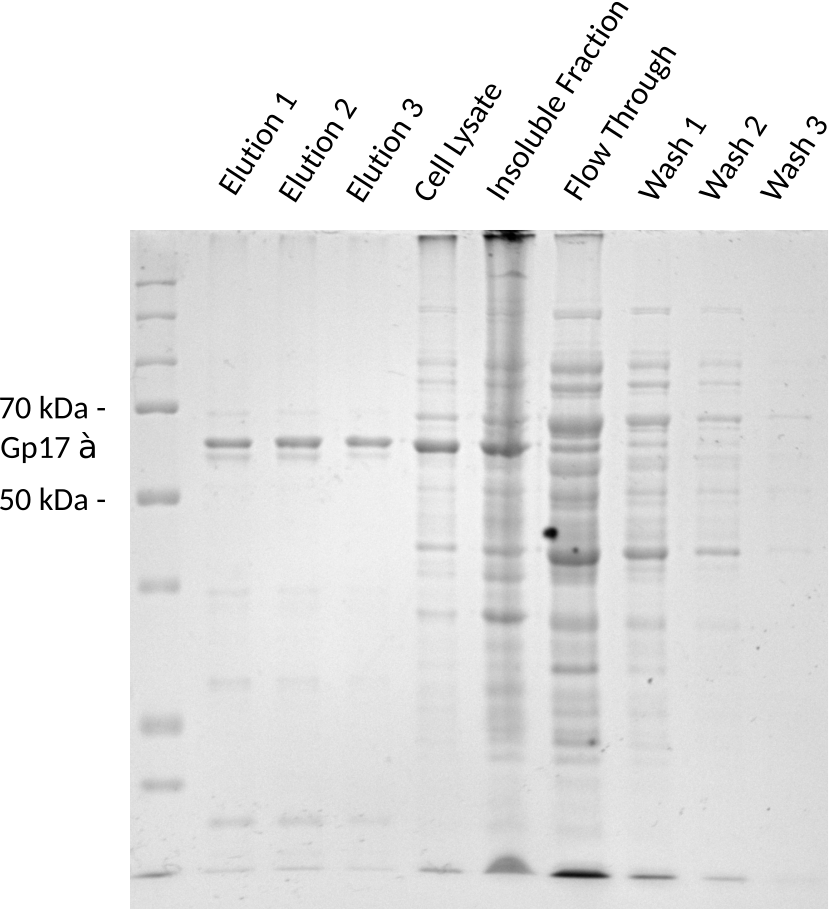
The SDS-page protein gel in figure 1 shows that Gp17(62 kDa) could be successfully purified from the cell lysate. Some impurities can still be observed, but can potentially be removed by additional washing steps or by using a higher quality columns for purification. The cell lysate shows that the protein is successfully overexpressed. A portion of Gp17 is in the insoluble fraction. The flow-through contains very little Gp17 compared to the cell lysate, which confirms that the tag binds to the column. The wash steps confirm that the protein is gradually cleaned up from other protein components and that Gp17 is not washed off the column. To conclude, this gel shows that the cleavable Twin-Strep tag can effectively be used to purify Gp17. A protein concentration of 407 ug/ml, 417 ug/ml and 225 ug/ml was determined for each of the three elution steps respectively by a BCA assay.
Cleavage of the Twin-Strep tag from the Purified Gp17
After purification of the protein, the Twin-Strep tag was cleaved off by factor Xa that recognized the cleavage site between the Gp17 protein and the tag. Different concentrations of factor Xa protease (Promega 1mg/ml) and incubation times were tested in order to determine the optimal conditions that ensure maximal cleavage of the tag and minimal off-target cleavage of the purified proteins. The protein concentration of Gp17 was adjusted to 0.25 ug/ul in a total volume of 40 ul with 0.025 ug/ul (1U), 0.005 ug/ul (0.2 U) or 0.00125 ug/ul (0.05 U) of factor Xa respectively. After 2h, 5h, 7h and 16h of incubation with factor Xa, a 2 ul sample was taken and immediately prepared for SDS page with Leammli buffer and stored at – 20 °C for analysis.

In the first lane of the gel (figure 2), the purified Gp17 protein without factor Xa was loaded. For all concentrations of factor Xa protease, the cleaved tag appears as small band at the bottom of the gel. This confirms the functionality of the introduced factor Xa cleavage site. A band is observed between the Gp17 and the cleaved tag for the cleavage with 0.025 ug/ul factor Xa. This indicates that the high protease concentration leads to off-target effects. The Gp17 amino acid sequence itself does not contain any factor Xa cleavage sites that could potentially be recognized by the protease.
For a factor Xa concentration of 0.005 ug/ul, the intensity of the cleaved tag reaches is maximum after 7h. No off-target cleavage could be observed. After 16h, two bands start appearing for the cleaved tag. This is probably due to the fact, that factor Xa cleaves its recognition site mostly after the C-terminus, but can also cleave it with lower efficiency after the N-terminus. Therefore, longer incubation times can lead to a second cut in the cleaved tag.
For the lowest concentration of factor Xa, the intensity of the cleaved tag is much lower than for the others. This leads to the conclusion that the optimal conditions for the cleavage is incubation with 0.005 ug/ul for 7h. However, these conditions might change for another protein, so it is recommended to repeat this test for each newly purified protein.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal AgeI site found at 1087
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI site found at 1594
Illegal BsaI.rc site found at 604
| None |
