Part:BBa_K3183012
CD27L Endolysin Residues 1-179 Targetting C. difficile Optimized for E. coli
This part contains the N-terminal CD27L1-179. This endolysin has previously been shown to be specific to C. difficile. This endolysin works by cleaving the bond between N-acetylmuramic acid and L-alanine in the peptidoglycan1. (Fig. 1), breaking the cell's peptidoglycan and compromising the structural integrity of its cell wall. Two X-ray crystal structures were solved for the N-terminal domain 2.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Characterisation
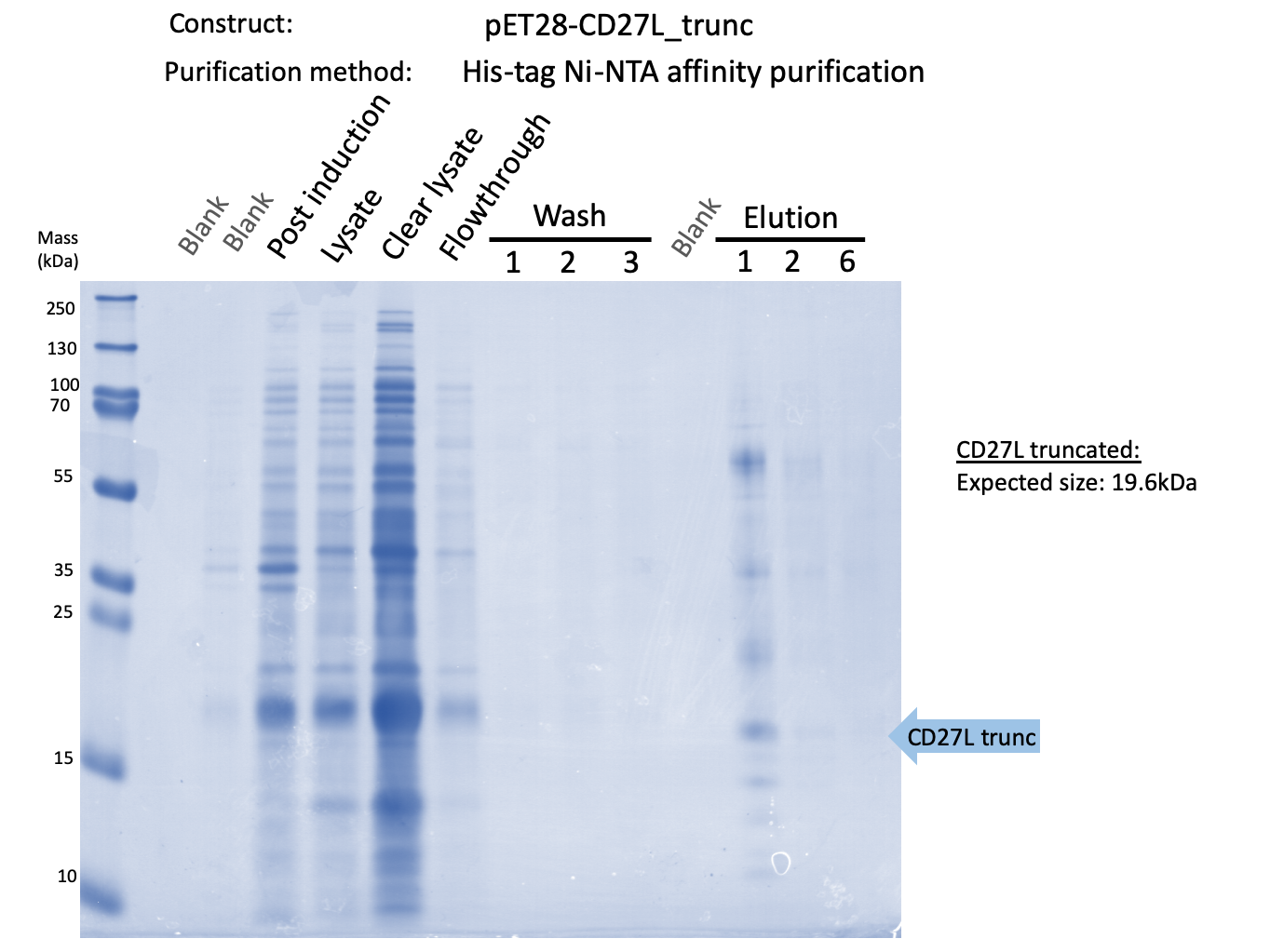
We expressed endolysin in the pET28A vector following difficulties with Dundee's CD27L1-179 endolysin BBa_K895005. We were able to achieve strong expression under the pET28A system (Fig. 3).
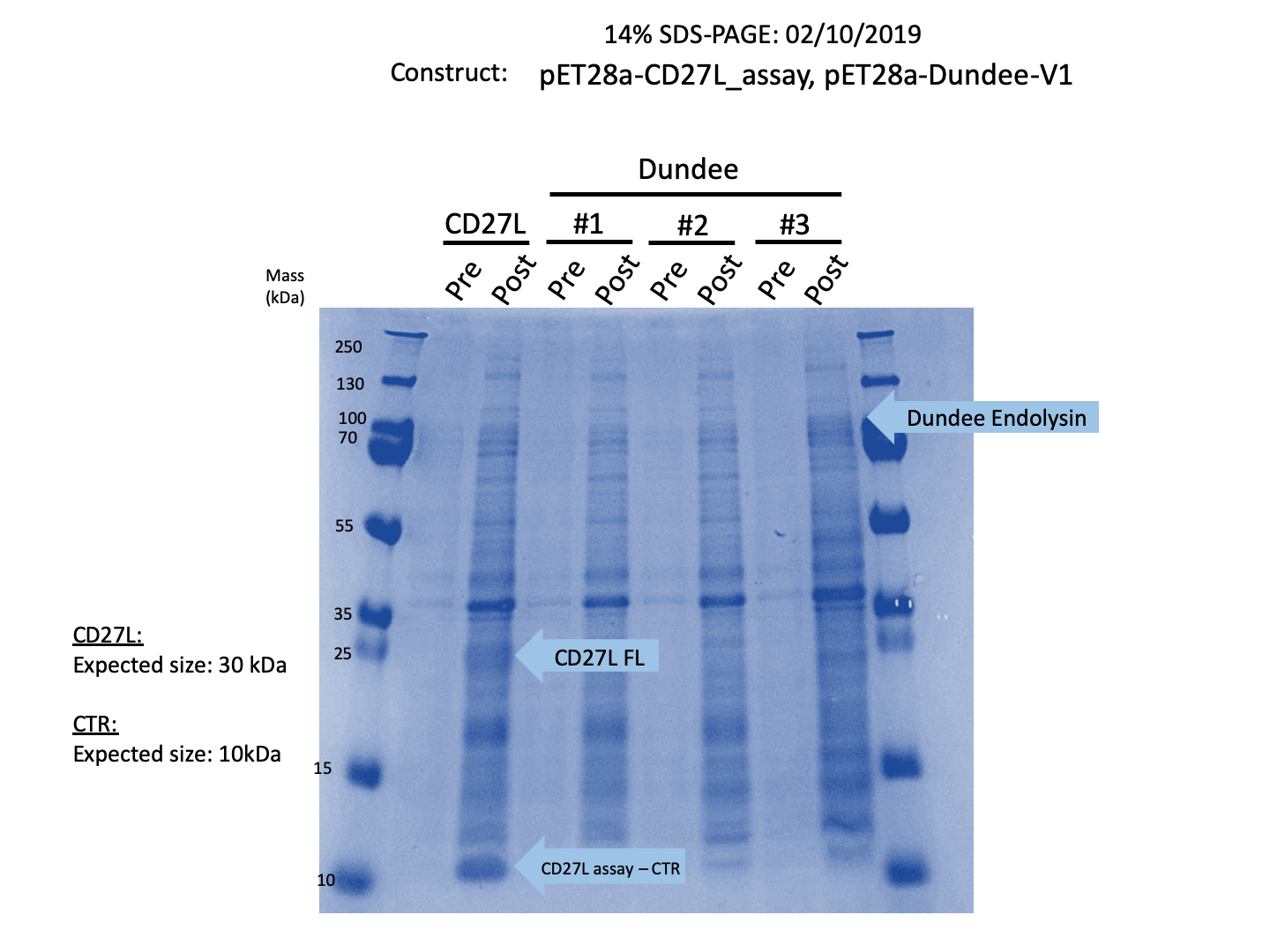
Due to safety concerns, our endolysin killing assays could only be carried out on on Bacillus subitlis, a Category 1 bacteria as opposed to Clostridium difficile, a Category 2 bacteria. B. subtilis has a similar composition of peptidogylcan cell wall to C. difficile2, allowing us to quantify killing data. We wanted to see if the truncated endolysin gave a measurable increase in lytic activity on B. subtilis as has been previously described in the literature2. As seen in Figure 3, there is a substantial decrease in OD600 over time relative to the negative controls for CD27L, and a slight decrease in OD600 of B. subtilis for CD27L1-179.
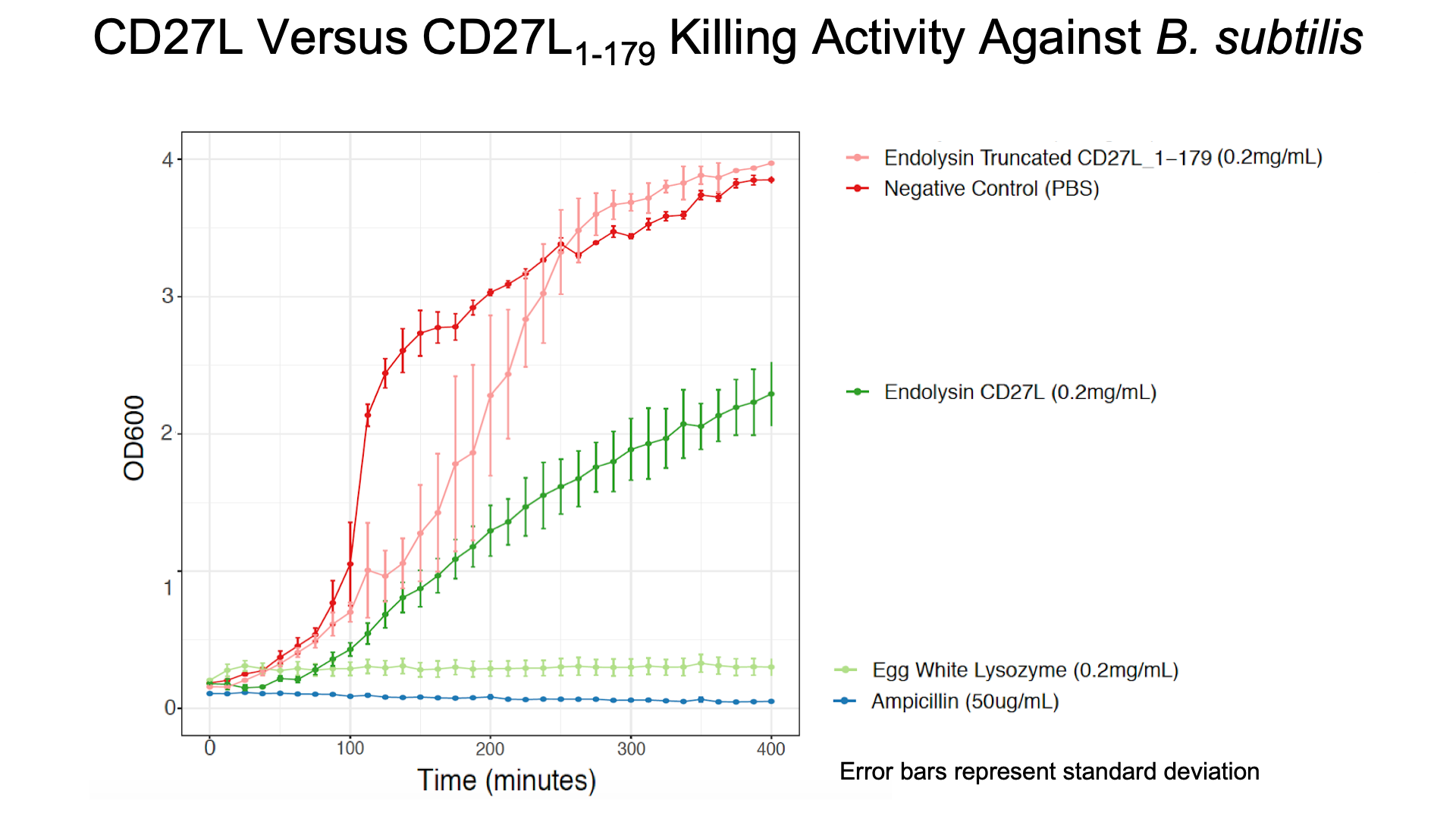
The truncated CD27L1-179 shows decreased growth relative to the negative control; however, log-phase growth resumes after 150 minutes. CD27L endolysin results in decreased growth during the first 100 minutes, and growth thereafter appears to be linear. This may point to inhibited log-phase growth in subsequent generations of B. subtilis following CD27L exposure.
Part Improvement of BBa_K895004
A fundamental crux of the ProQuorum system is the expression of the endolysin which is responsible for the actual lysis and consequent killing of the pathogenic C. difficile. Thus, there is a clear need for an endolysin that fulfills the criteria of being:
1. optimally expressed
2. optimally secreted and
3. optimally effective against C.difficile
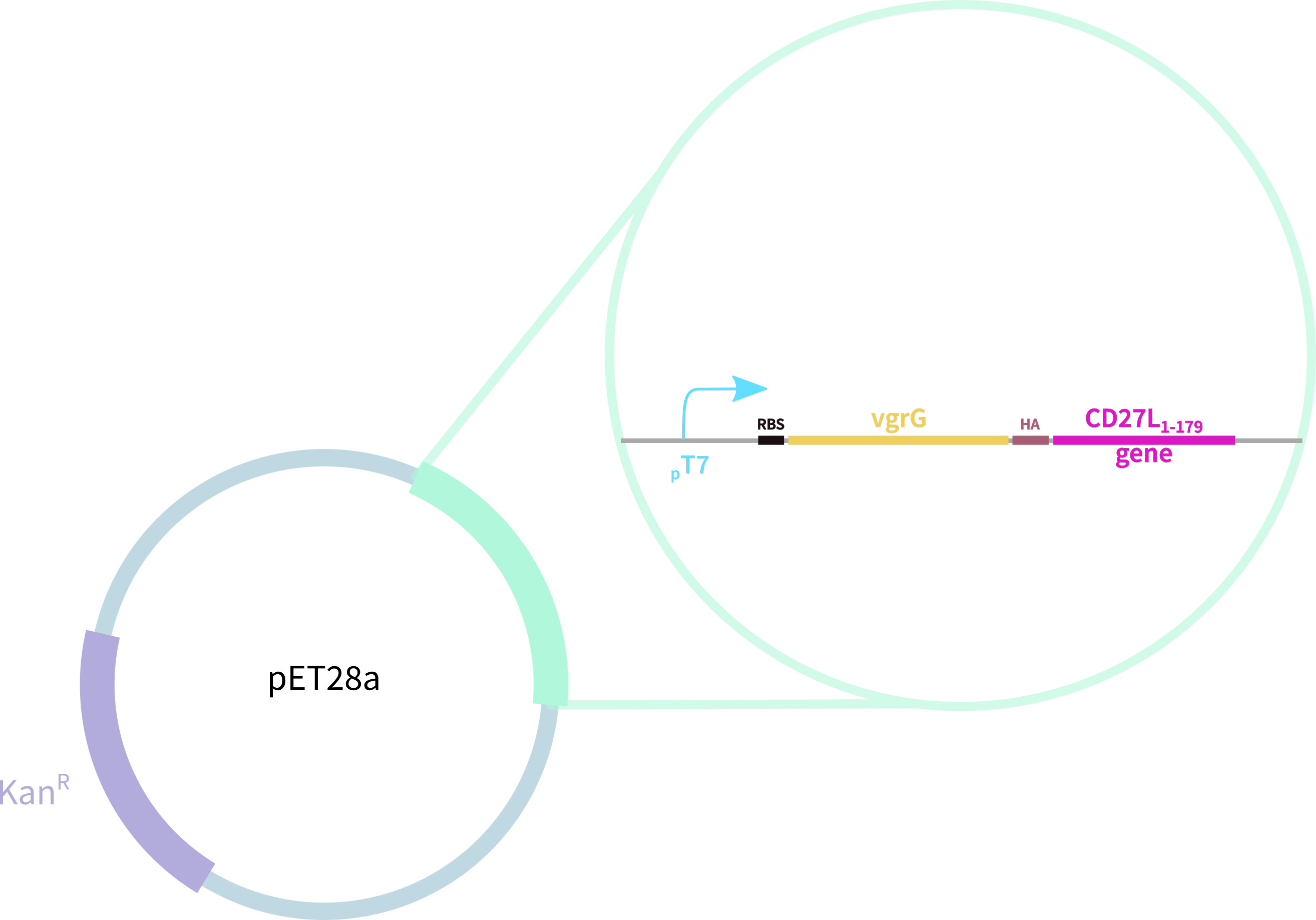
Thus, we redesigned the BBa_K895005 part submitted by the 2012 Dundee iGEM team, which consists of:
1. truncated form of the CD27L endolysin, shown to increase both efficacy and host range2
2. Type VI secretion protein derived from S. typhimurium
3. Hemagglutinin (HA) Tag for Western Blotting
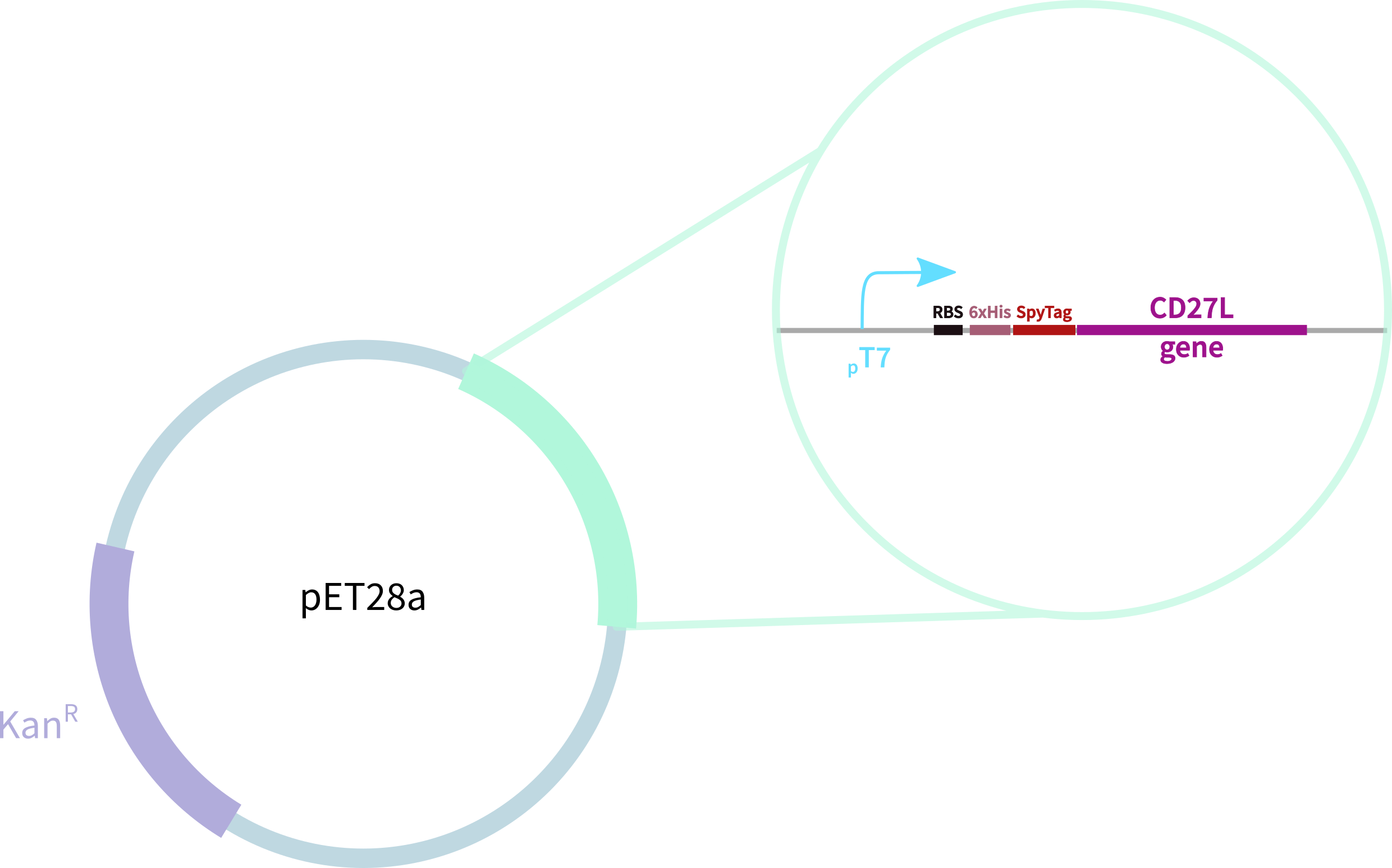
In contrast, our novel part BBa_KK3183200 (CD27L) encodes:
1. full-length form of the CD27L endolysin
2. SpyTag for purification, oligomerisation, and other SpyCatcher applications
3. 6-His tag for easy purification
Incorporating both of these parts into pET28A, our expression vector, we planned on testing the relative efficacies of B.subtilis killing as per our killing assays. Thus, we followed our generic pipeline of:
1. transformation of both constructs into E.coli (BL21 (DE3)-RIPL Competent E.coli)
2. miniprep + sequencing to verify successful transformation
3. induction of expression with IPTG
Thus, we sought out the endolysin in an existing part made by the 2012 iGEM Team Dundee. However, despite identical growth and induction conditions, expression of the Dundee 2012 iGEM endolysin was unsuccessful, even in triplicate, as shown in Figure 1. In contrast, expression of the CD27L_Assay part was successful and quantitatively verified via both mass spectrometry and BCA assay, as in the Results page.
Thus, to solve this issue, we decided to attempt expression of only the truncated endolysin from the Dundee 2012 Biobrick, without its substantially large Type VI secretion tag. We added 6His and SpyTag to the N-terminus to allow for detection (via SpyCatcher gel-shift assays) and purification. This resulted in BBa_K31830201 (CD27L1-179</sib<). Upon analysis, there is evident expression of the truncated endolysin as seen in Figure 2. However, given identical conditions of growth and induction, there appears to be greater expression of the full-length endolysin, which may perhaps indicate greater stability.
As a further improvement, we decided to measure the killing efficacy of the CD27L_Assay endolysin on B. subtilis (our surrogate target) as no killing data was provided for the Dundee 2012 BBa_K895005 part. As seen in Figure 3, there is a substantial decrease in OD600 over time relative to the negative controls.

The truncated CD27L1-179 shows decreased growth relative to the negative control; however, log-phase growth resumes after 150 minutes. CD27L endolysin results in decreased growth during the first 100 minutes, and growth thereafter appears to be linear. This may point to inhibited log-phase growth in subsequent generations of B. subtilis following CD27L exposure.
Reference
1. Mayer, M. J., et al. “Molecular Characterization of a Clostridium Difficile Bacteriophage and Its Cloned Biologically Active Endolysin.” Journal of Bacteriology, vol. 190, no. 20, 2008, pp. 6734–6740., doi:10.1128/jb.00686-08.
2. Mayer, M. J et al. “Structure-based modification of a Clostridium difficile-targeting endolysin affects activity and host range.” Journal of bacteriology vol. 193,19 (2011): 5477-86. doi:10.1128/JB.00439-11
3. Dunne, Matthew et al. “The CD27L and CTP1L endolysins targeting Clostridia contain a built-in trigger and release factor.” PLoS pathogens vol. 10,7 e1004228. 24 Jul. 2014, doi:10.1371/journal.ppat.1004228
4. Twetman, Svante, et al. “Scanning Electron Microscopic Study of Streptococcus Mutans BHT Lysed by Lysozyme.” European Journal of Oral Sciences, vol. 93, no. 1, 1985, pp. 23–29., doi:10.1111/j.1600-0722.1985.tb01304.x.
| None |