Part:BBa_K3110051
lldRO1-J23117-lldRO2 Strong RBS YebF-IL12
This part contains the lldRO1-J23117-lldRO2 attached to a strong RBS, YebF-IL12-FLAG and a double terminator.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 78
Illegal NheI site found at 101 - 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Usage and Biology
The regulatory element lldR binds to the lldRO1 and lldRO2 (the operator regions) and inhibits transcription. J23117 is a promoter intercalated between the operators. lldR dimer represses the transcription, possibly by forming a DNA loop which doesn’t allow the RNA polymerase to bind to the promoter. Upon binding of L-lactate to lldR, this transcriptional suppression is lost and the lldR complex with L-lactate remains bound to LldRO1, acting as a transcriptional activator.
IL12 is an anti-inflammatory cytokine involved in the regulation of cell-mediated immune responses. Through a complex signalling cascade, IL12 activates our immune cells to reduce tumor growth. Its production is under the control of the lactate operon's promoter, lldPRDp, which has been optimised to respond specifically to lactic acid concentrations in the milieu of colon cancer cells. YebF is a secretory protein present in E. coli. We have attached the IL12 sequence in the C-terminus of YebF so that IL12 also gets secreted out of E. coli along with YebF. A FLAG tag is attached downstream to YebF so that its secretion outside the cells can be detected using FLAG antibodies.
Characterization of the construct
The lactate sensitive promoter lldPRDp was characterised and sfGFP was observed to give better fluorescence intensity under O1PO2 than when it was placed under the control of a constitutive promoter. So, IL12 was placed under the control of O1PO2. This should give specificity to the production of IL12 only at desired concentrations of lactate. A secretory protein YebF was conjugated to IL12 so as to facilitate its secretion outside the cells once it is produced in the cancer microenvironment. The FLAG tag was attached downstream to detect the secretion of IL12 outside the cells.
Experimental Procedure
- We chose BL21(DE3) as our chassis and transformed the plasmid containing our construct in BL21(DE3). We cultured them in LB (with Chloramphenicol at a concentration of 34 ng/ul) for 12 hours at 37°C, 200 r.p.m. in two separate conical flasks.
- One of them was induced with L-lactate at an OD of 0.4 while the other was left uninduced.
- After 12 hours, the culture medium and the cell pellet were then taken for western blot after undergoing sample preparation which included the concentration of supernatant using a 10 kDa MWCO filter.
- The samples were then run on a 15% polyacrylamide gel and followed by transferring onto a PVDF membrane with a pore size of 0.2 µm. The membrane was then probed with the help of FLAG primary antibodies (raised in mouse) and a secondary anti-mouse antibody conjugated with HRP.
- For imaging, luminol-peroxide based solution was used, as our secondary antibody was conjugated with HRP.
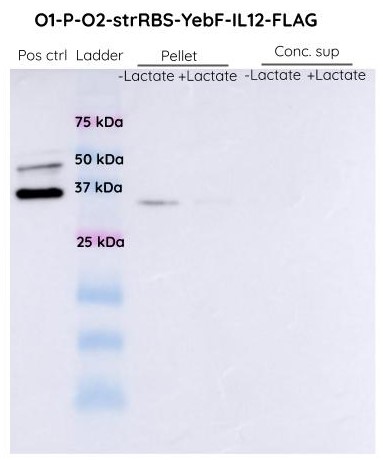
Results
In the case of constitutive promoter upstream to YebF-IL12, we can see a band at around 74 KDa (expected size) in the pellet fraction. The band is present both in the control and test samples. However, no band was observed in the supernatant.
In the case of O1PO2 upstream to YebF-IL12, we can see a band at around 74 KDa in the supernatant and cell pellet both with and without lactate induction.
The SDS-PAGES were not very conclusive and we decided to proceed with Western Blot analysis to confirm if the band corresponded to YEBF-IL12.
We didn't observe any band at around 74 KDa. Hence we can conclude that the protein is nor being produced only. We suspected that there might have been SOEing errors in the steps of cloning and this requires further investigation.
| None |