Part:BBa_K3016100:Experience
This experience page is provided so that any user may enter their experience using this part.
Please enter
how you used this part and how it worked out.
Applications of BBa_K3016100
Use by Aalto-Helsinki 2019
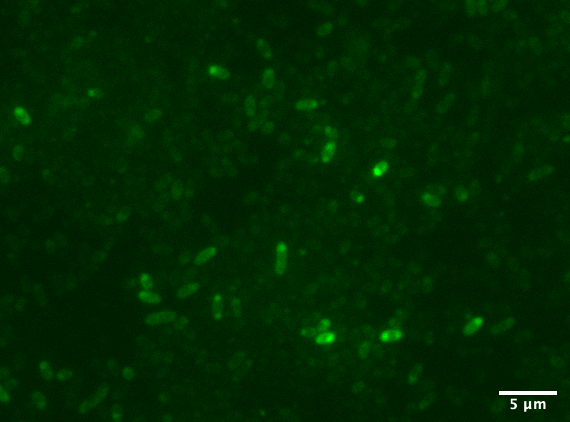
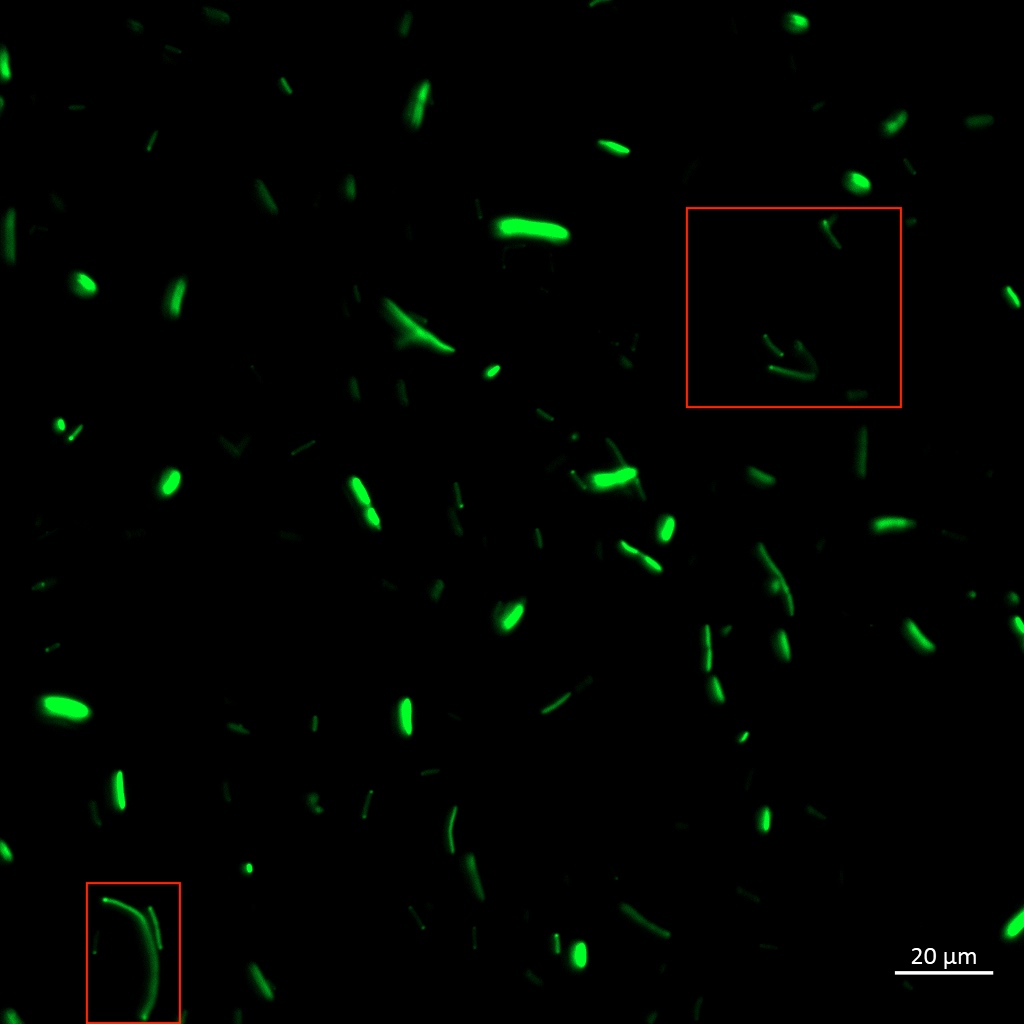
Aalto-Helsinki 2019 characterized this part by combining it with YGFP (BBa_K3016600), a slow-bleaching GFP variant, creating a composite TorA-YGFP part (BBa_K3016200) to test protein translocation into Vibrio natriegens' and Escherichia coli DH5a's periplasm.
In the images above we see Vibrio natriegens and Escherichia coli DH5a cells expressing TorA-YGFP. Note the polar localisation of fluorescence. This can be a sign of periplasmic localisation of YGFP under osmotic pressure (Sochacki et al., 2011) or inclusion body formation (Jong et al., 2017).
To be certain of successful periplasmic translocation, a cell fractionation experiment was performed on Vibrio natriegens. The periplasmic fraction of TorA-YGFP expressing cells was extracted (PureFrac-protocol) and placed under UV light, image below. The presence of YGFP in the periplasmic fractions can be seen clearly.
These results indicate that the Vibrio natriegens' TorA signal peptide successfully translocates proteins into the periplasm of Vibrio natriegens, and possibly even of Escherichia coli.
References:
Alanen, H. I., Walker, K. L., Suberbie, M. L. V., Matos, C. F., Bönisch, S., Freedman, R. B., ... & Robinson, C. (2015). Efficient export of human growth hormone, interferon α2b and antibody fragments to the periplasm by the Escherichia coli Tat pathway in the absence of prior disulfide bond formation. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research, 1853(3), 756-763.
Jong, W. S., Vikström, D., Houben, D., de Gier, J. W., & Luirink, J. (2017). Application of an E. coli signal sequence as a versatile inclusion body tag. Microbial cell factories, 16(1), 50.
Sochacki, K. A., Shkel, I. A., Record, M. T., & Weisshaar, J. C. (2011). Protein diffusion in the periplasm of E. coli under osmotic stress. Biophysical journal, 100(1), 22-31.
User Reviews
UNIQ8bd51443a65ddae5-partinfo-00000000-QINU UNIQ8bd51443a65ddae5-partinfo-00000001-QINU