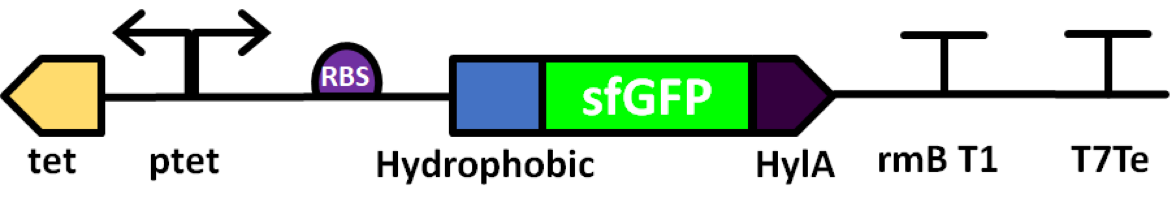
Part:BBa_K2906002
sfGFP with C-Terminal secretion signal (HylA)
Superfolder GFP also known as sfGFP is a GFP-derived green fluorescent protein. (Pédelacq et al., 2006). GFP is a protein isolated from the jellyfish Aequorea Victoria that exhibits green fluorescence when exposed to light in the blue to the ultraviolet range (Prendergast and Mann, 1978; Tsien, 1998). A series of mutations performed by Pédelacq et al. obtained a GFP variant able to rapidly fold and mature. This ultimately also lead to enhanced fluorescence intensity (Frenzel et al., 2018). This composite part is made of sfGFP (BBa_K1321337) with a hydrophobic tag BBa_K2906007 and a C-Terminal secretion signal (HylA) BBa_K554002 to secrete the protein into the cytoplasm. It is expressed by a pTet promoter BBa_R0040. Below we discuss our reasoning behind these choices and our quantitative and qualitative findings. Unfortunately, this part was unable to express colour and unable to be secreted into the media.
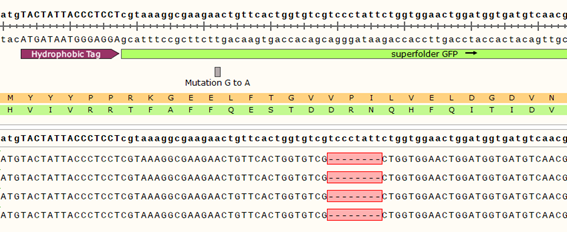
Note: Multiple cloning attempts were not able to generate sfGFP with HylA construct. Due to time constraints, experiments were performed with a mutated version of the sfGFP C-terminal construct. The mutation obtained was an 8 bp deletion from position 31 to 39 bp of sfGFP, causing a frame-shift mutation. However, this deletion is found 49-57 bp away from the start codon, located immediately upstream of the hydrophobic tag. See Figure below.
Contents
HylA Signal Peptide
α-hemolysin has been previously characterised to provide efficient secretion of active correctly folded proteins to the culture supernatant in E. coli (Fraile et al., 2004). This hemolysin secretion system is a well-characterised type I secretion system which provides a one-step translocation, from the bacterial cytoplasm to the extracellular medium without a periplasmic intermediate of tagged proteins. The secretion signal does not become cleaved after trafficking across the bacterial membrane (Gentschev, Dietrich and Goebel, 2002; Ruano-Gallego et al., 2019).
Hydrophobic Tag:
Since we aimed at the creation of new hair dyes that would not damage the cuticle of hair, we did not want our designed coloured protein-based dyes to infiltrate the cuticle as this will lead to cuticle opening and weaken the hair itself. Therefore, both of our secreted variants also contained a novel hydrophobic tag (BBa_K2906007)
Characterisation:
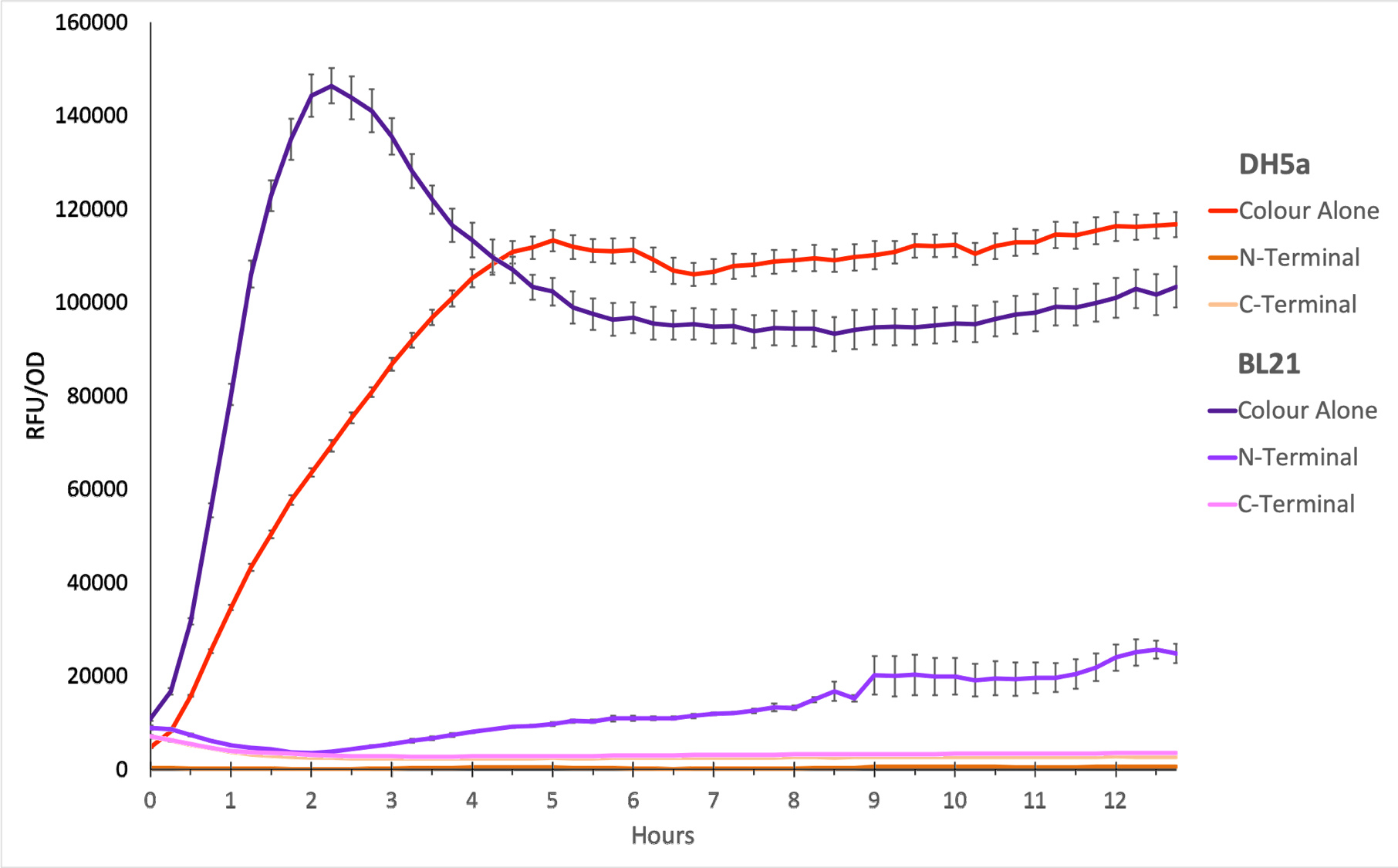
To show whether this part worked, we decided to measure fluorescence, normalised by OD. Optical Density values were measured at 600 nm. We grew the cells to an OD600 of ~0.6 and induced them with 100 nM of anhydrotetracycline. Then, they were loaded in triplicates into a 96-well plate and left overnight. For OD values, the blank was LB media, and for RFU the blank was E. coli TOP10 since it does not express any colour. The values were individually normalised by dividing RFU/OD and then averaged to plot the mean against time. An RFU value of 0 corresponds to baseline E. coli TOP10 measurements.
Figure 1. The plot shows the mean RFU/OD from three replicates of each construct expressed in E. coli DH5⍺ and BL21(DE3). The OD was measured at 600 nm and GFP fluorescence was measured at Ex ƛ 485, Em ƛ 510, every 15 minutes for 13 hours. The RFU values were normalised by the OD and the triplicates averaged. All values have been blank-corrected. A total of 52 recordings were made per well, with three wells per construct. The C-Terminal construct does not show fluorescence in E. coli cells.
Qualitative Data:
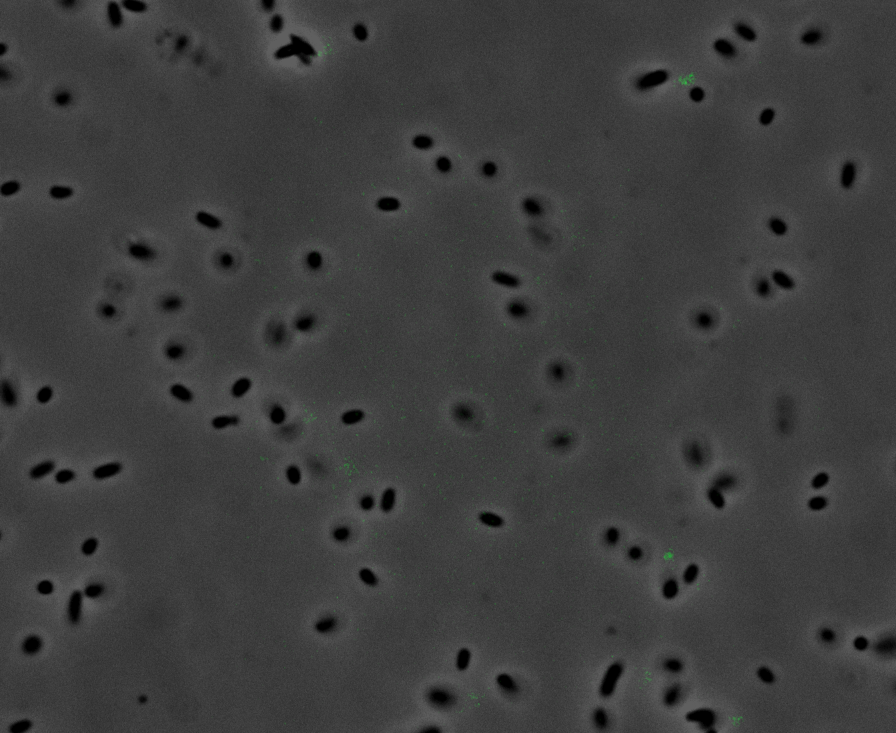
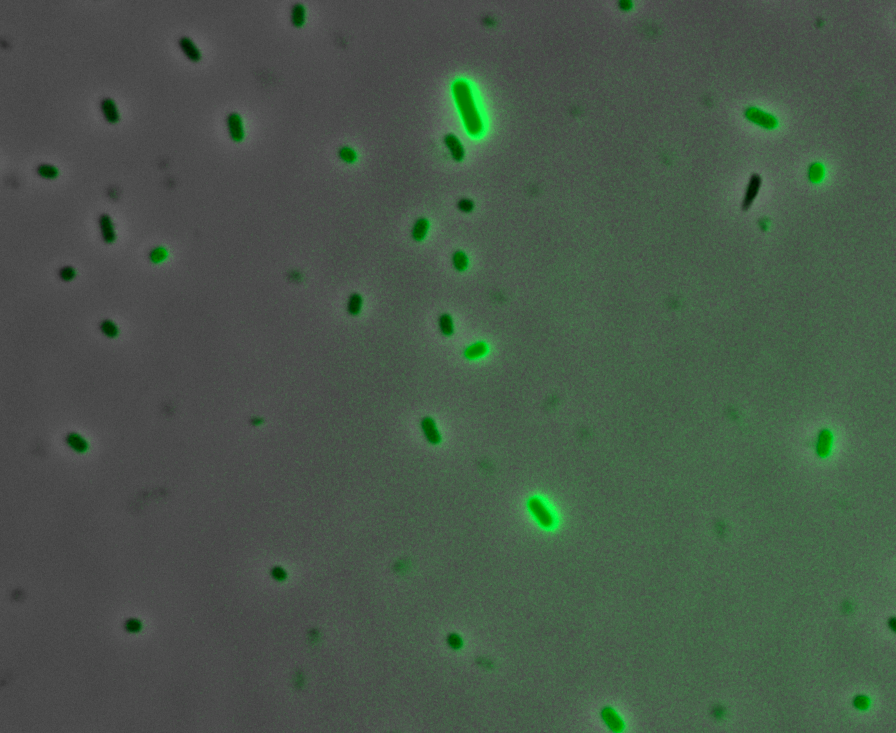
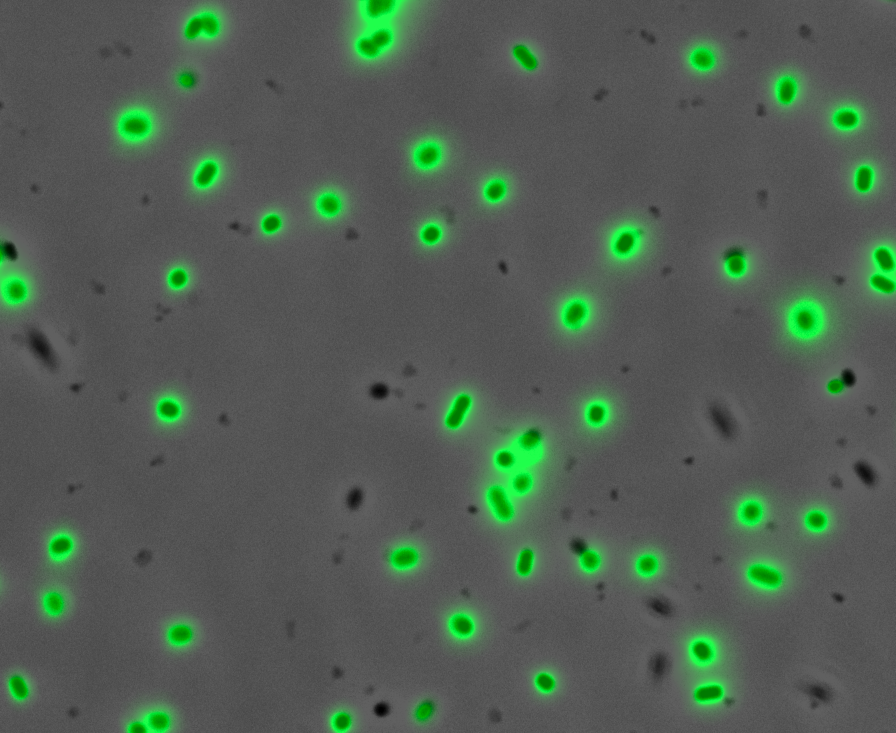
Fluorescence Microscopy: After quantifying the data for sfGFP we decided to perform fluorescence microscopy. We did this since the experiments above show that there is a trace amount of sfGFP detected through fluorescence spectrometry (higher than the negative control, but no colour was seen. This would allow us to see the percentage of bacteria in a sample producing colour, if any, as well as some phenotypic characteristics of the bacteria with the construct. Below you can see three images, the negative control, which was E. coli TOP10, showing very limited background GFP fluorescence, sfGFP with HylA (BBa_K2906002) showing limited amounts of fluorescence, and BBa_K2906000, which clearly expresses sfGFP.
Figure 2. Fluorescent microscopy overlaid images of phase contrast and GFP filter. a) Negative control (E. coli TOP10) showing very slight background readings for fluorescence. b) sfGFP + HylA (BBa_K2906002) showing very limited fluorescence in some bacteria and some background noise, this shows that fluorescence is not intense. c) BBa_K2906000 showing appropriate levels of fluorescence in most bacteria. The images above are composite overlaid images made from phase contrast and GFP-filter captures. They were processed using ImageJ.
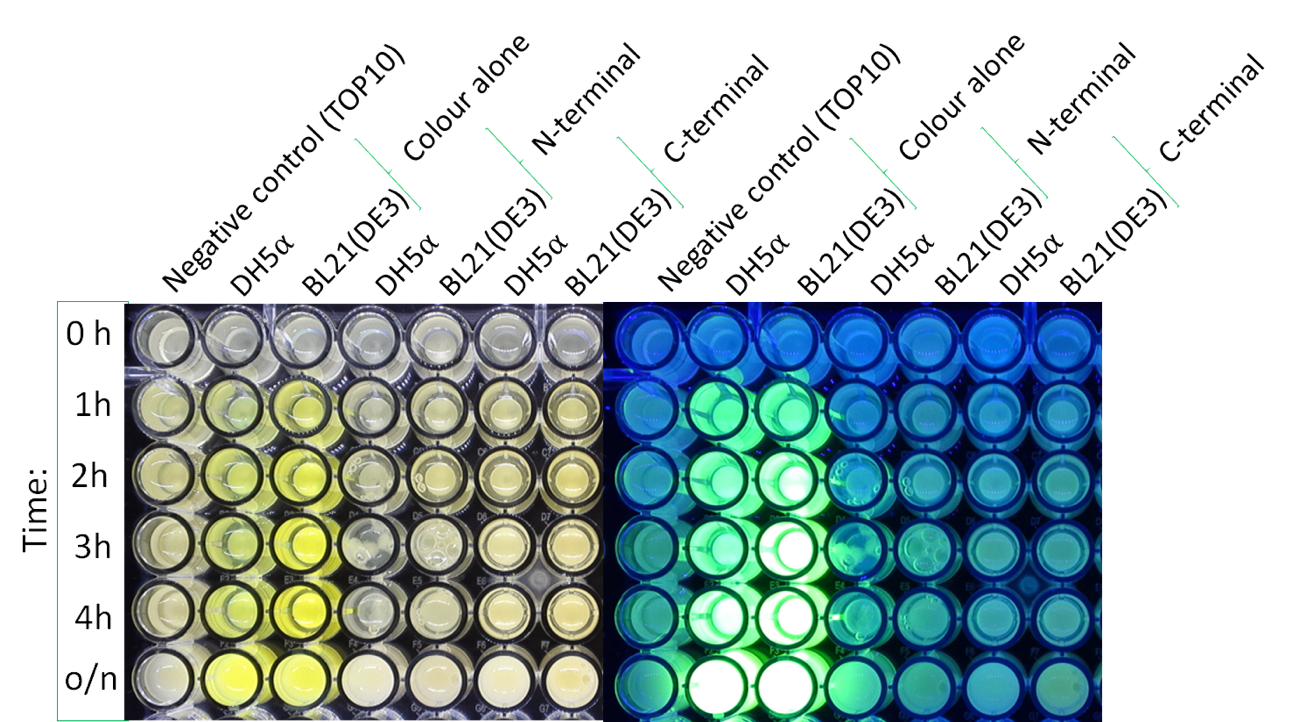
Colour:
The image below shows the visible colour of the pellet obtained under normal light and UV light. This was done at five different time points after induction with 100 nM of anhydrotetracycline. sfGFP with HylA is labelled ''C-Terminal'' and can be seen present in both DH5a and BL21.
References:
Fraile, S., Muñoz, A., De Lorenzo, V. and Fernández, L. A. (2004) ‘Secretion of proteins with dimerization capacity by the haemolysin type I transport system of Escherichia coli’, Molecular Microbiology, 53(4), pp. 1109–1121. doi: 10.1111/j.1365-2958.2004.04205.x.
Frenzel, E., Legebeke, J., Van Stralen, A., Van Kranenburg, R. and Kuipers, O. P. (2018) ‘In vivo selection of sfGFP variants with improved and reliable functionality in industrially important thermophilic bacteria’, Biotechnology for Biofuels. BioMed Central Ltd., 11(1). doi: 10.1186/s13068-017-1008-5.
Gentschev, I., Dietrich, G. and Goebel, W. (2002) ‘The E. coli alpha-hemolysin secretion system and its use in vaccine development.’, Trends in microbiology, 10(1), pp. 39–45. doi: 10.1016/s0966-842x(01)02259-4.
Pédelacq, J. D., Cabantous, S., Tran, T., Terwilliger, T. C. and Waldo, G. S. (2006) ‘Engineering and characterization of a superfolder green fluorescent protein’, Nature Biotechnology, 24(1), pp. 79–88. doi: 10.1038/nbt1172.
Prendergast, F. G. and Mann, K. G. (1978) ‘Chemical and Physical Properties of Aequorin and the Green Fluorescent Protein Isolated from Aequorea forskålea†’, Biochemistry, 17(17), pp. 3448–3453. doi: 10.1021/bi00610a004.
Ruano-Gallego, D., Fraile, S., Gutierrez, C. and Fernández, L. Á. (2019) ‘Screening and purification of nanobodies from E. coli culture supernatants using the hemolysin secretion system’, Microbial Cell Factories. BioMed Central Ltd., 18(1). doi: 10.1186/s12934-019-1094-0.
Tsien, R. Y. (1998) ‘THE GREEN FLUORESCENT PROTEIN’, Annual Review of Biochemistry. Annual Reviews, 67(1), pp. 509–544. doi: 10.1146/annurev.biochem.67.1.509.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal SapI.rc site found at 115
| None |