Part:BBa_K2578600
Expression of MymT under constitutive promoter
Metallothioneins (MTs) are small cysteine-rich proteins made of 61-68 amino acids which can be found in a broad range of organisms, including both eukaryotes and prokaryotes. The MTs are expressed as intracellular protein. [1]
MTs are mainly responsible for metalloregulation in cells of living organisms. The rich Cys domains in MTs allow the non-covalent binding of trace metals such as cadmium, lead, copper and mercury, etc. [2,3]
MT family contains four members, MT1 - MT4. Each member shares similar properties but slightly different affinity in binding different metals. Among most studies, it was found that cadmium, lead and mercury, which are some most toxic metal ions displayed the highest binding affinity with MTs. [3] These results show that MTs are capable in the use of bioremediation of toxic heavy metals.
[1] N Thirumoorthy, KT Manisenthil Kumar, A Shyam Sundar, L Panayappan, Malay Chatterjee (2007). Metallothionein: An overview. World journal of gastroenterology, ISSN 1007-9327
[2] Almaguer-Cantú V1, Morales-Ramos LH, Balderas-Rentería I. (2011) Biosorption of lead (II) and cadmium (II) using Escherichia coli genetically engineered with mice metallothionein I. Water Sci Technol. 2011;63(8):1607-13.
[3] Dziegiel, P., Pula, B., Kobierzycki, C., Stasiolek, M., Podhorska-Okolow, M. (2016), Metallothioneins in Normal and Cancer Cells, Springer International Publishing, DOI: 10.1007/978-3-319-27472-0
MymT is a small prokaryotic copper metallothionein discovered in Mycobacterium tuberculosis. It is believed that the protein may help the bacterium survive copper toxicity, though deleting the gene had no effect on pathogenicity in mice. It can bind up to 7 copper ions but has a preference for 4-6.
A form of MymT was already in the registry at the start of our project (BBa_K190020) but it was not codon optimised to E. coli and we saw no evidence that it had been used. Team iGEM16_Oxford has codon optimised it and added a C terminal his tag for purification. In part (BBa_K1980003) we added a C terminal sfGFP to show if the protein is expressed in E. coli and obtained provisional data, using fluorescence lifetime microscopy, suggesting copper-binding in vivo.
The followings are a part of description about BBa_K1980002 given by iGEM16_Oxford :
FLIM
We discovered a paper by Hötzer et al(2) that described how His-tagged GFP can be quenched by a copper ion binding to this His tag leading to a reduction in the fluorescence lifetime (the time the fluorophore spends in the excited state before returning to the ground state by emitting a photon.) They speculated that this could potentially be used as a in vivo copper assay.

As we had His-tagged our chelator-sfGFP constructs we were curious to see if this technique could be applied to our parts to measure copper chelation in vivo by our parts. We believe that two possibilities were likely:
- Copper chelation by the chelator reduces the free copper concentration inside the cell meaning that less binds to the His tag and the fluorescence lifetime will be greater than a His-tagged sfGFP control
- Copper chelation by the chelator would allow additional quenching if copper was bound within the quenching radius of the fluorophore leading to a reduction in fluorescence lifetime compare with a sfGFP control
Lacking access to a fluorescence lifetime microscope ourselves we contacted Cardiff iGEM who had a FLIM machine in their bioimaging unit.
We sent Cardiff iGEM our parts MymTsfGFP in pBAD and pCopA CueR sfGFP (as a control) in live MG1655 E. coli in agar tubes. Cardiff grew them overnight in 5ml of LB with 5uM copper with and without 2mM arabinose.
The imaging unit spread each strain on slides and measured the fluorescence lifetime of three areas on each slide.
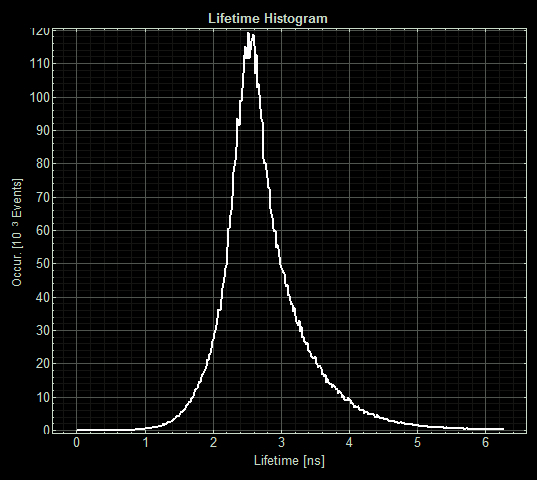
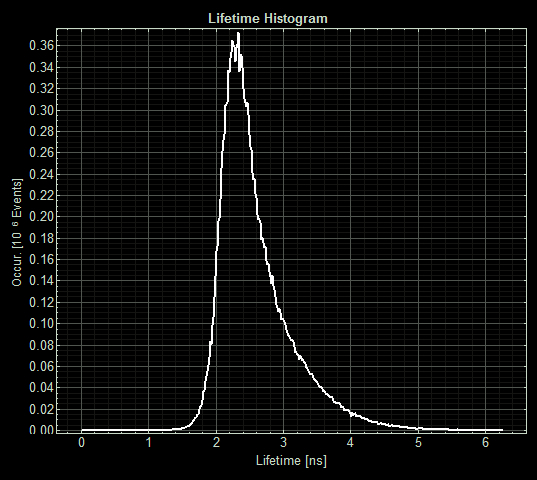
(Acquisition parameters: using the x63 water immersion objective with excitation at 483nm (71% intensity, pulse rate 40MHz) and emission via a BP500-550 filter. Scan resolution at 512 x 512 pixels at pixel size of 0.26 microns/pixel, 1AU pinhole. Counts of >1000 per lifetime recording.)
FLIM images from one section of each slide:

As expected the pCopA CueR sfGFP control was fluorescent, with and without arabinose, with the mean fluorescence lifetime a consistent 2.6ns.
When MymTsfGFP was induced the mean lifetime decreased to 2.3ns. As MymTsfGFP is was observed to be reliably expressed and because MymT is a small copper cluster separated form sfGFP by a small linker we believe that this represents additional quenching of the fluorphore by MymT-bound copper showing in vivo copper chelation.
Copper absorption test
When put in a composite part, the MymT gene can produce MymT in the transformed cell. We test the ability of our MymT gene inserted bacteria on copper absorption by growing it for 0, 2 and 4 hours in two different medium with different copper concentrations. We use empty vector bacteria as a control
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 7
Illegal NheI site found at 30 - 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
| None |