Part:BBa_K1688008
ModLac laccase with His-tag (inc RBS and J23110 promoter)
ModLac is a modified laccase (D439A/M510L CueO) with N-His6 tag attached to it via a linker sequence.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 7
Illegal NheI site found at 30 - 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 310
- 1000COMPATIBLE WITH RFC[1000]
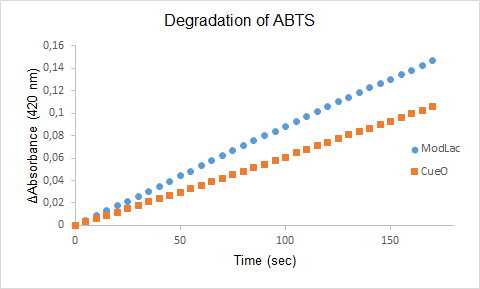
Figure 1. The degradation of ABTS by the lysate of ModLac and the lysate of CueO over time.
Usage and biology
Laccases (originally from Chinese lacquer tree sap) are multicopper oxidases that are employed in various industries where they take part in beer maturation, textile dyeing, and enzymatic biofuel cells. Due to their broad specificity and ability to oxidize aromatic compounds their application in bioremediation is a topic under investigation.
Design Notes
The laccase we chose is a bacterial laccase, CueO, a laccase from E. coli. The CueO laccase was then modified with a double mutation (D439A/M510L) that has been proven to increase the enzymatic activity (Kataoka K et al. 2012). A polyhistidine-tag was also added at the N-terminus so that it could be purified easily. Restriction sites were also added before and after the 6xhis-tag so that it could be removed.
Characterization
The aim with doing modifications of this laccase (CueO) was to improve the enzymatic activity of the protein.
The enzymatic activities of the laccases was measured using ABTS. ABTS is a commonly used substrate when evaluating reaction kinetics of specific enzymes. Due to its reduction potential, it acts as an effective electron donor. Since we are working with laccases, that have the capability to oxidize ring structure, ABTS is a suitable substrate. ABTS will donate an electron to reduce molecular oxygen. The oxidized ABTS has a different absorption spectrum and the reaction can thus be observed in a spectrophotometer.
The enzyme assays of the lysates with ABTS showed a significant difference in enzyme activity between CueO and the mutant CueO, also known as ModLac. The modifications of the laccase that was made increased the enzymatic degradation of ABTS. This is confirmed by our results (See Figure 1).
References
Kataoka, K, Kogi H, Tsujimura S, Sakurai T. 2012. Modifications of laccase activities of copper efflux oxidase, CueO by synergistic mutations in the first and second coordination spheres of the type I copper center. Faculty of pure and applied science , University of Tsukuba,
| n/a | ModLac laccase with His-tag (inc RBS and J23110 promoter) |