Part:BBa_K1499401
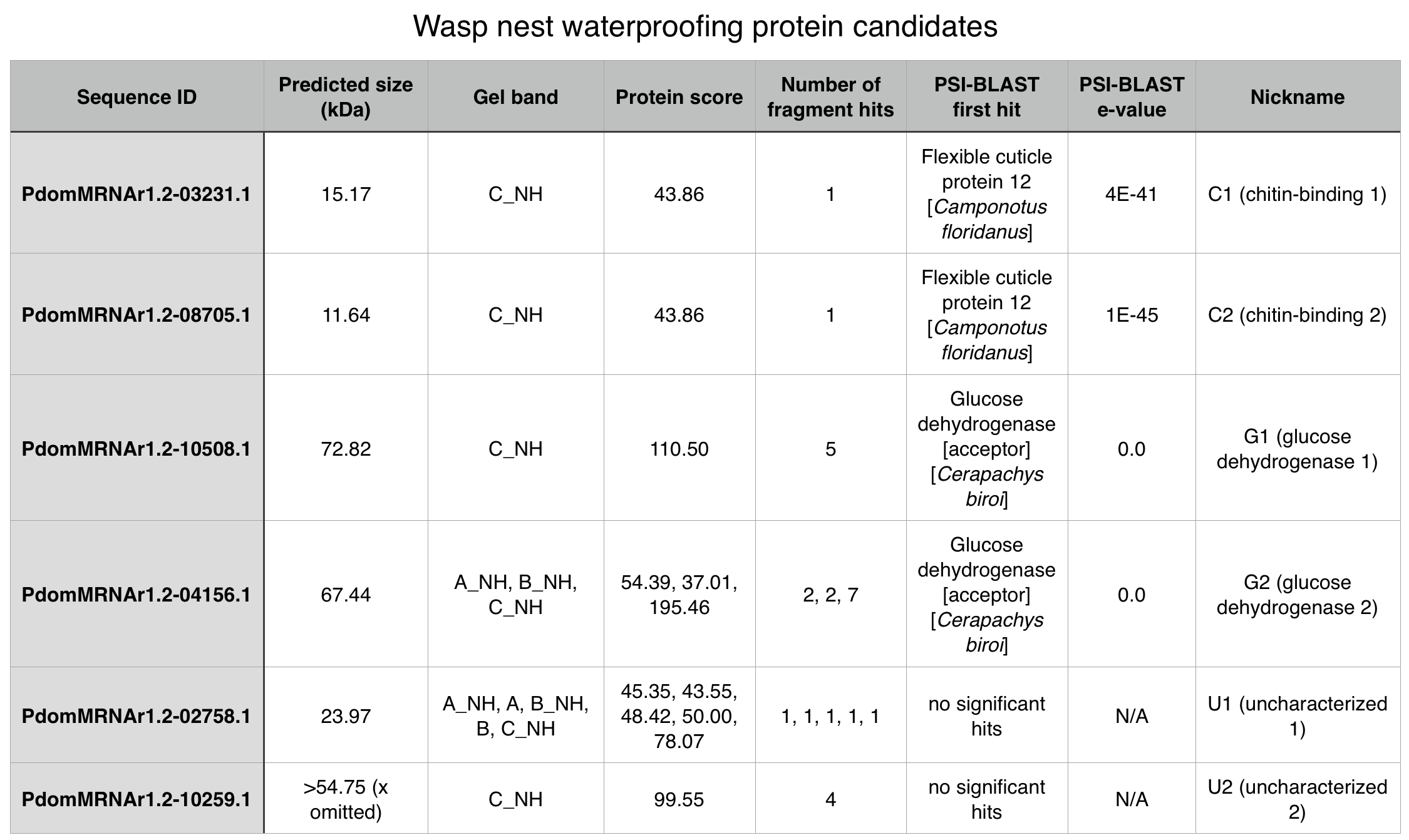
Chitin-binding wasp protein 2 (PdomMRNAr1.2-08705.1)
For more information on this project, visit our wiki: http://2014.igem.org/Team:StanfordBrownSpelman/Material_Waterproofing
Usage and Biology
Paper wasps of the genus Polistes are well known for their ability to construct nests composed of a tough, fibrous paper-like material. Studies have shown that the wasps build these nests by collecting cellulose from plants in the environment, chewing it up, and mixing with a protein-rich oral secretion that helps strengthen and cement the cellulose into nest paper[1]. Interestingly, these nests are also hydrophobic, which leads us to believe there may be a single protein in wasp saliva responsible for coating and waterproofing the nest paper.
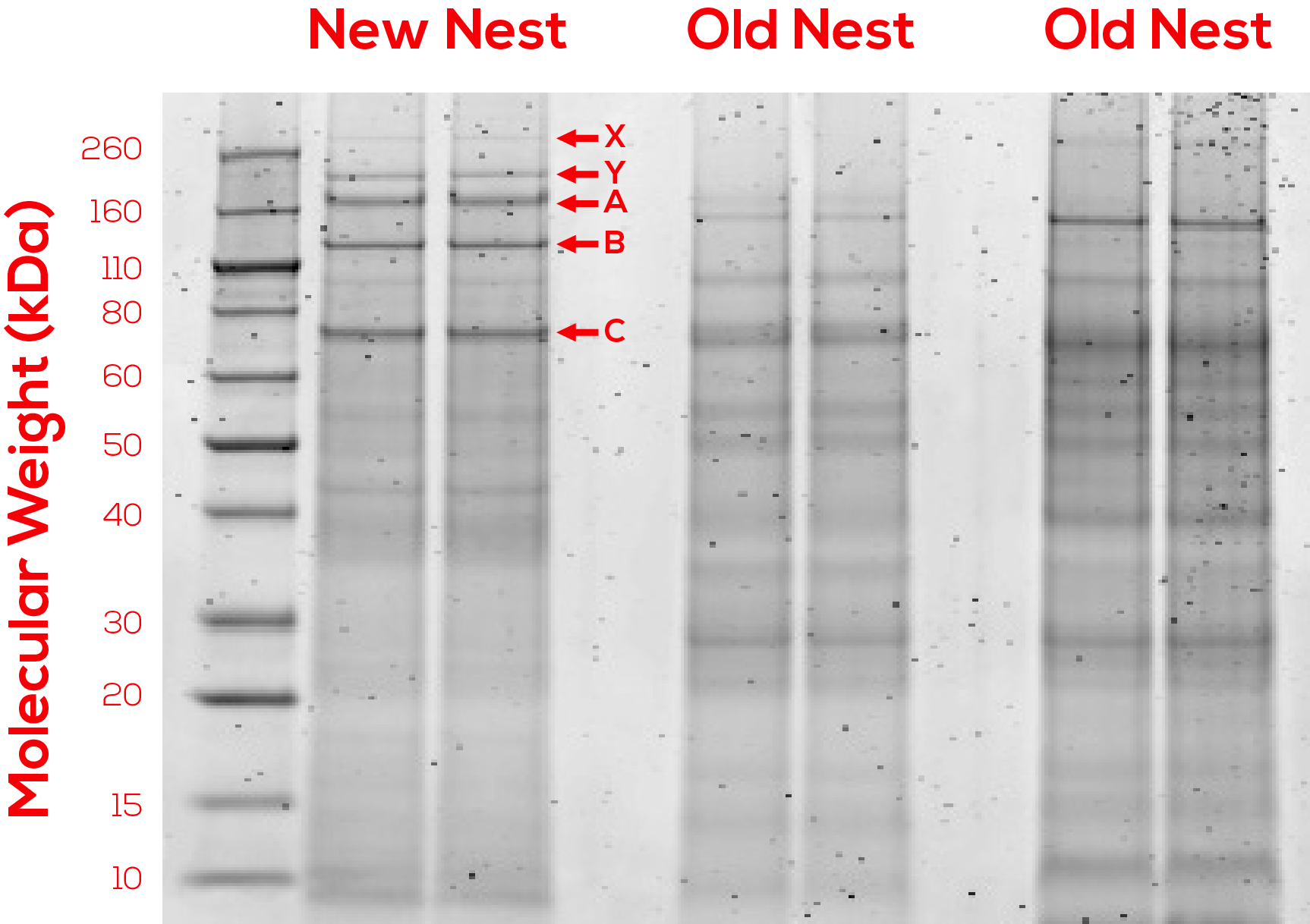
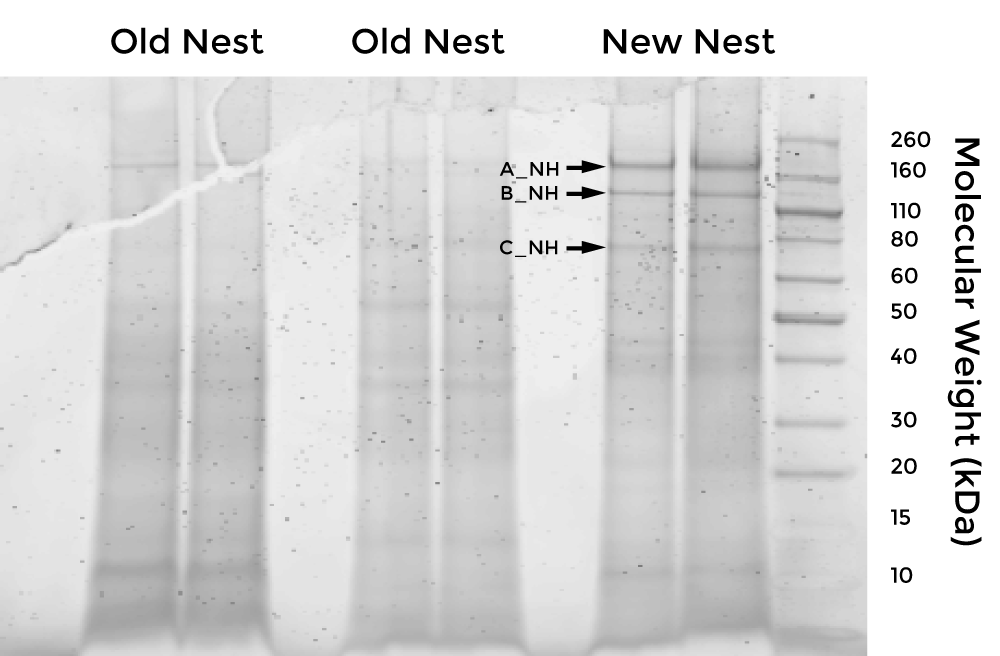
In an attempt to identify and characterize this protein, we collected samples of nests constructed by Polistes dominula and extracted total protein. After resolving the protein extract on polyacrylamide gels, we excised dominant bands and sent them to the Wessel lab at Brown University for peptide mass fingerprinting.
After analyzing the peptide mass fingerprinting results, we selected this protein as a candidate for the hypothesized waterproofing protein, due to PSI-BLAST results indicating that it may have a chitin-binding domain (chitin is a close chemical relative of cellulose).
The full suite of proteins that we investigated, along with additional information about this protein is viewable in the following table (Figure 3).
Results
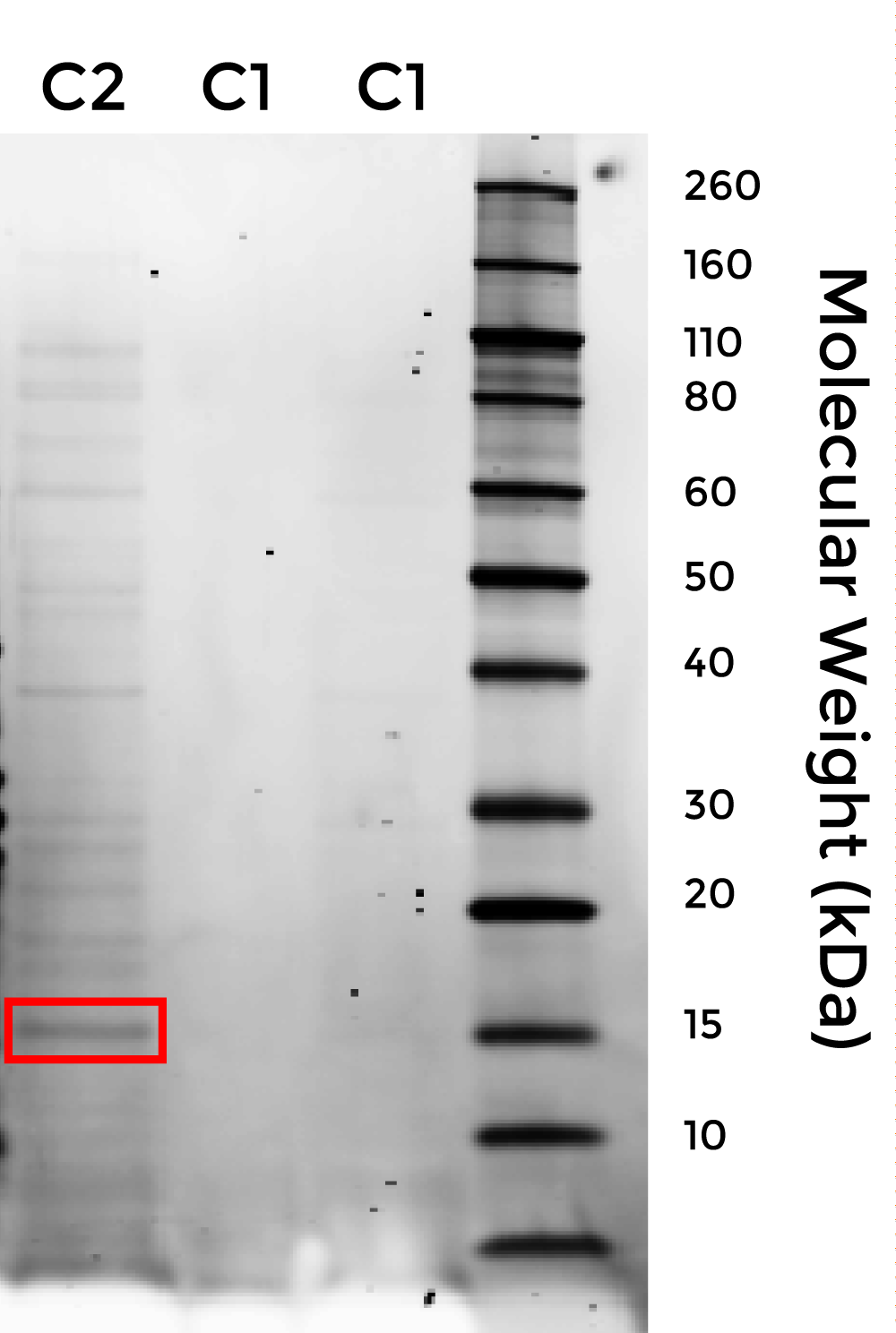
To purify this protein from cell lysate, we used the New England Biolabs Chitin Magnetic Beads protein purification kit, which isolates proteins that bind to chitin-coated magnetic beads. The following gel image shows that PdomMRNAr1.2-08705.1 (nicknamed C2) was successfully purified with the chitin magnetic beads, suggesting that it exhibits chitin-binding activity. Pending cellulose-binding and waterproofing assays, C2 is a candidate for waterproofing both fungal mycelium and bacterial cellulose.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
References
1. Kudo, K et al. (2000) Amino acid composition of the protein in pre-emergence nests of a paper wasp, Polistes chinensis (Hymenoptera, Vespidae). Insectes soc. 47(4):371-375.
| None |