Part:BBa_K1021001
nptII (neomycin phosphotransferase)
The nptII gene encodes neomycin phosphotransferase, which confers resistance to geneticin, kanamycin, and related antibiotics.
Usage and Biology
- Cornell iGEM demonstrated the functionality of this part via transformation (in vector pNG) into C. heterosporus. The fungal transformants:

- This part has been used in the construction of composite and generator parts BBa_K1021015, BBa_K1021029, BBa_K1021027, and BBa_K1021032.
- This part has been improved by codon optimization for E. coli in BBa_K2195002. A promoter and an RBS were added to the codon optimized gene in that part.
This gene has also been used by team UNIK copenhagen 2015. Since the sequence we used is only almost a twin, we had to add it to the registry for the purpose of making a composite biobrick (BBa_K1825005 and BBa_K1825006). Nevertheless, here is our experience using the nptII-gene to transform a new chassis organism.
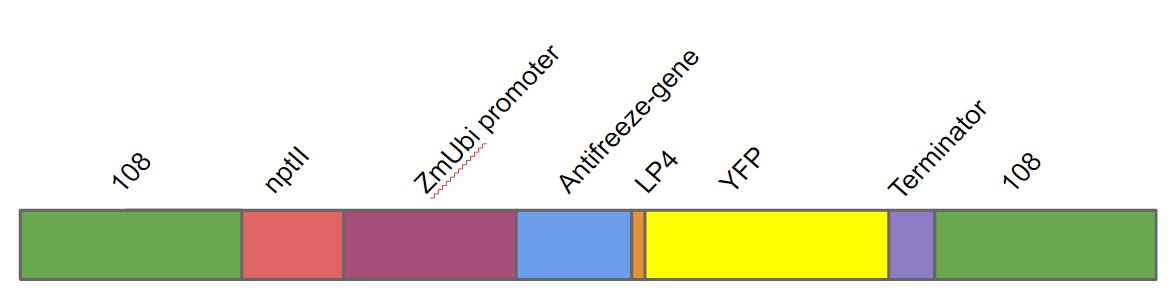
The gene was a part of a larger construct used for transforming moss Physcomitrella patens. We transformed this gene into Physcomitrella patens as a part of a larger DNA construct (fig. 1). This DNA construct contained in the following order. A homologous region to the 108 locus on the moss genome (to ensure stable integration into P. patens genome), the nptII-resistance gene driven by the 35s cauliflower mosaic virus, the Zea mays ubiquitin promoter driving the antifreeze protein (AFP) linked to yellow fluorescent protein (YFP) with the LP4 linker sequence, terminator and lastly another 108 region.
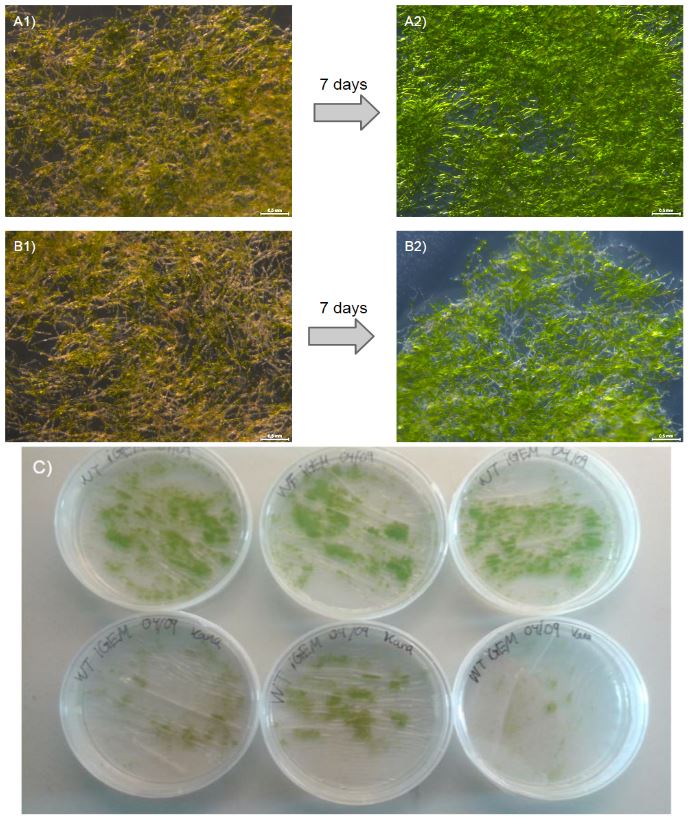
Our moss was able to grow from single transformed protoplasts to full clumps on media containing kanamycin (50 mg/ml), which suggests that the nptII-resistance cassette provides P.Patens with resistance to kanamycin (fig. 2).
To further validate this, we outlined an additional experiment where we grew wild type (WT) moss on either nonselective PhyB-media (3 plates) or PhyB-media containing kanamycin 50 mg/ml (3 plates). After 7 days, there was a visible difference in growth between WT on nonselective media and WT on kanamycin plates (fig. 3). The WT moss planted on kanamycin containing plates grew very little or not at all and is seen under the microscope as withering (fig. 3 B2). This is a stark contrast to our transformed moss that was able to grow from protoplasts to full clumps and the more vibrant WT without kanamycin (fig. 3 A2). This experiment validates the function of the the neomycin phosphotransferase II gene in P. patens.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 796
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 629
- 1000INCOMPATIBLE WITH RFC[1000]Illegal SapI.rc site found at 478
Illegal SapI.rc site found at 688
| None |