Part:BBa_I2032
T7 RNA polymerase
Gene 1 coding region from bacteriophage T7 encoding an RNAP polymerase (RNAP). This single-protein RNAP recognizes a 21 bp consensus promoter sequence and is orthogonal to E. coli RNAP.
Contribution: Team Aboa 2022
Here we provide background information on the T7 RNA Polymerase from the literature.
Background
T7 RNA Polymerase (T7 RNAP) is a single subunit protein originating from T7 bacteriophage that catalyzes RNA synthesis. It is known for its high processivity and great specificity to the T7 promoter. T7 RNAP was isolated for the first time in 1969 from E. coli that was infected by T7 phage (Tunitskaya & Kochetkov, 2002).
T7 RNAP and the T7 system are used in a wide array of applications. Because of its simplicity, it is often used as a model when studying transcription mechanisms (Kochetkov et al, 1998). However, its use in eukaryotic cells is hindered by the need for posttranscriptional modifications and the transportation of the eukaryotic mRNA from the nucleus to the cytoplasm (Wang et al, 2018; Borkotoky & Murali, 2018).
Compared to the RNA polymerase in E. coli T7 has several advantages. It is able to elongate five times faster than the E. coli polymerase and has only one subunit compared to the multi-subunit polymerase in E. coli. It also has higher specificity to its promoter.
Structure
The primary structure of T7 RNAP is composed of 883 amino acid residues and its molecular weight is 98 092 Da (Kochetkov et al, 1998). The primary structure of T7 RNAP was first determined in the 1980s (Tunitskaya & Kochetkov, 2002).
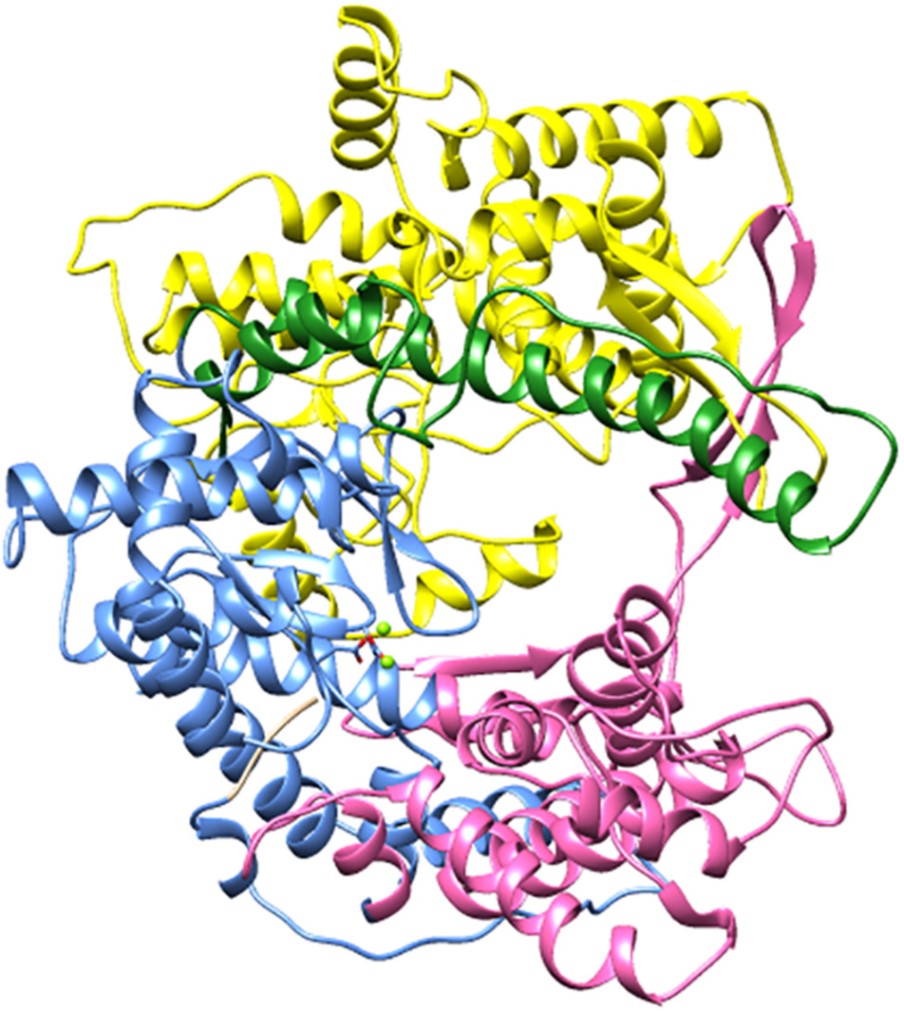
T7 RNAP has three subdomains that are described as its “thumb”, “palm”, and “fingers” due to their resemblance to the right hand (Tunitskaya & Kochetkov, 2002), seen in Figure 1. The thumb subdomain comprises the residues ~330–410, the fingers subdomain is ~541–737, and ~771–778. The palm domain has most of the catalytically important residues and comprises residues ~386–448, ~532–540, and ~788–838. The subdomains form a 60 Å long, 15-25 Å wide, and 25-40 Å deep cleft that acts as the binding site for the template DNA. The cleft can fit almost two turns of dsDNA. (Kochetkov et al, 1998; Tunitskaya & Kochetkov, 2002.)
There are three structural motifs, A, B, and C, situated inside the cleft that are conserved through most single subunit nucleotide polymerases (Kochetkov et al, 1998). Motifs A and C that have invariant residues of aspartic acid are observed in all DNA and RNA polymerases. They are situated in the palm subdomain. Motif B in the fingers subdomain is conserved in all RNAPs and some DNAPs and contains invariant residues of lysine, tyrosine, and glycine. (Tunitskaya & Kochetkov, 2002.)
The N-terminal end of the T7 RNAP has a role in sequence-specific promoter binding and opening the duplex DNA (Borkotoky & Murali, 2018).
References
Borkotoky, S., Murali, A. (2018) The highly efficient T7 RNA polymerase: A wonder macromolecule in biological realm. International Journal of Biological Macromolecules, 118.
Kochetkov, S., Rusakova, E., Tunitskaya, V. (1998). Recent studies of T7 RNA polymerase mechanism. FEBS Letters, 440.
Tunitskaya, V., Kochetkov, S. (2002) Structural–Functional Analysis of Bacteriophage T7 RNA Polymerase. Biochemistry (Moscow), 67.
Wang, W., Li, Y., Wang, Y., Shi, C., Li, C., Li, Q., Linhardt, R. (2018) Bacteriophage T7 transcription system: an enabling tool in synthetic biology. Biotechnology Advances, 36.
Improved Part
Improved part: BBa_K2443037 submitted by Lethbridge iGEM 2017
Rational behind improvements: BBa_I2032 encodes exclusively for the coding region of T7 RNA polymerase. In order to improve it and incorporate it into our Next vivo system we have Codon optimized for use in E. coli and attached a N-terminal hexahistidine tag with a serine glycine linker for easy purification. We have also optimized our T7 RNA polymerase to be overexpressed in BL21-Gold (DE3) cells by putting it under the control of a T7 promoter (BBa_I719005), RBS (BBa_B0034) and double terminator (BBa_B0015).
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
| None |