Help:Terminators/Measurement/Caitlin Conboy
< Back to Terminator Measurement Help

|
Caitlin Conboy, as a member of Drew Endy's lab, constructed and characterized transcriptional terminators BBa_B0020-BBa_B0025 and characterized transcriptional terminators BBa_B0011-BBa_B0015. |
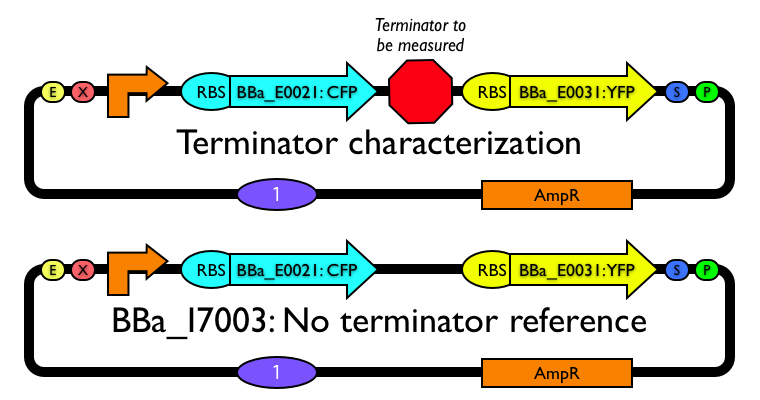
A series of terminator characterization constructs was built using BioBrick® standard assembly (Table 1). Each construct encodes ECFP and EYFP on a polycistronic message, separated by the transcriptional terminator of interest. The ECFP and EYFP (BBa_E0021 and BBa_E0031, respectively) used in this study were derived from Clontech expression vectors and include the native ribosome binding site ([http://orders.clontech.com/clontech/techinfo/vectors_dis/pECFP.shtml], [http://orders.clontech.com/clontech/techinfo/vectors_dis/pEYFP.shtml], [1]). The wild-type lac promoter (BBa_R0010) drives expression of the construct. All constructs were built on pSB1A1) in E. coli strain DH5a (Figure 1). Characterization constructs were built for six terminators and their reverse counterparts (BBa_B0010-BBa_B0015 and BBa_B0020-BBa_B0025).
We measure termination efficiency by inserting each terminator between the coding regions for CFP and YFP on a polycistronic message and measuring the fluorescence ratio of the two fluorophores by flow cytometry. A control construct lacking an internal terminator (BBa_I7003) was used to determine a baseline YFP to CFP fluorescence ratio. Output from this construct was assumed to represent zero percent termination efficiency. Additional constructs expressing ECFP only (BBa_I7000) and EYFP only (BBa_I7001) from the same promoter served as positive controls for fluorescent protein expression.
Sample Preparation
Overnight cultures were inoculated from plated colonies in 5 ml of LB medium with 50 μg/ml ampicillin. Samples were grown at 37°C. In late log phase (OD600nm=~1.5-2), 1 ml of culture was pelleted, resuspended in 1 ml PBS, and stored on ice for analysis by flow cytometry.
Flow cytometry was performed using a FACS Aria machine equipped with two lasers, providing excitation at 405 nm for CFP and 480 nm for YFP. CFP fluorescence was detected using a 475/85 filter (i.e., 475 +/-43 nm). YFP fluorescence was detected with a 530/30 filter (i.e., 530 +/-30nm).
Analysis
Population Average Termination Efficiency: Average terminator read-through was derived from the mean fluorescence ratio of YFP/CFP, normalized to the ratio of the no-terminator control construct (BBa_I7003). The analysis makes the assumption that the average YFP/CFP fluorescence ratio is correlated with the ratio of PoPSin/PoPSout. Termination efficiency is thus defined as one minus the terminator read-through:
Termination efficiency = 1 - (mean YFP/mean CFP*2.96)
Results and discussion
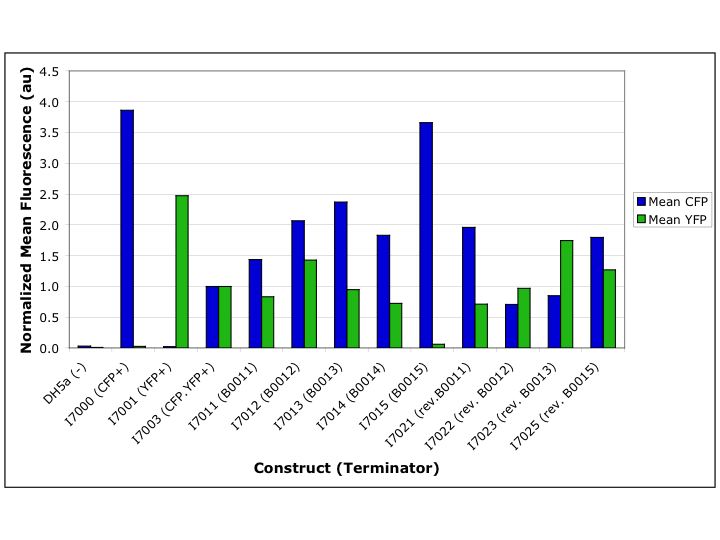
In this study, we measured a wide range of termination efficiencies among our set of terminators, from a high of 98.4% to a low of –106.0% (Table 2). Of the single terminators, BBa_B0013 had the highest forward termination efficiency (60.0%) but the lowest reverse efficiency (–106.0%). The sequence of BBa_B0013 was derived from the TE terminator of bacteriophage T7 and engineered to be bidirectional by inserting six adenine residues on the 5’ side of the hairpin sequence and mutating two additional residues to thymine in the sequence on the 3’ side of the hairpin. Compared to the wild-type TE terminator (BBa_B0012) rational engineering efforts in the design of BBa_B0013 improved the forward termination efficiency but made the reverse termination efficiency worse.
The other single terminator that we characterized was BBa_B0011, a terminator derived from the luxICDABEG operon of Vibrio fischeri and predicted to be bidirectional. BBa_B0011 had the highest combined forward and reverse termination efficiencies of the single terminators and was thus designated a “working” part in the Registry of Standard Biological Parts.
Two double terminators were also characterized: BBa_B0014, composed of BBa_B0012 and BBa_B0011; and BBa_B0015, composed of BBa_B0010 and BBa_B0012. BBa_B0014 had higher forward termination efficiency than either of its component terminators (60.4%). Due to difficulties in assembling the characterization construct, BBa_B0014 was not characterized in the reverse direction, nor was the single terminator BBa_B0010 characterized in either direction. BBa_B0015 was characterized as the most efficient forward terminator (98.4%) and a functional reverse terminator (29.5%).
The negative termination efficiencies measured for BBa_B0022 and Bba_B0023 may suggests one of two possibilities.
- The terminator parts have unexpected promoter activity.
- The insertion of a hairpin in the middle of the polycistronic transcript encoding CFP and YFP might stabilize the YFP transcript relative to that produced from the no terminator control, resulting in the production of more YFP per mRNA and thus a negative measured termination efficiency.
Variability in CFP expression between constructs and decreased CFP expression in the dual fluorophore constructs compared to the CFP only control suggest that high levels of transcript expression are saturating ribosomal translation capacity (Figure 2). Since our constructs are expressed from a high copy plasmid (estimated at 100-500 copies) and a relatively strong promoter, high levels of transcript levels are expected. However, such a translation bottleneck might affect the correlation of protein and mRNA levels and reduce the fidelity of our surrogate measurement for termination efficiency.
This work represents a initial attempt to characterize transcriptional terminators as standard biological parts. We attempted to measure the forward and reverse termination efficiencies of five terminators from the fluorescence ratio of two reporter genes, encoded upstream and downstream of the terminator of interest. Using reporter protein fluorescence to infer relative transcription levels assumes that the mRNA stability and translation rates are insensitive to variations in the terminator sequence and performance. This assumption was not tested in this study. A subsequent generation of terminator characterization experiments conducted by Jason Kelly partially addressed these concerns by flanking reporter coding regions with hairpins and RNase E sites to better ensure transcript stability and independence.
Areas for improvement
Subsequent characterization of transcriptional terminators should improve on this work in four ways.
- As discussed above, our characterization fails to address issues of mRNA stability and translation rates.
- The growth conditions used in this study were chosen somewhat arbitrarily and may not represent optimal characterization conditions. For example, the use of LB rather than a defined medium and (more significantly) the decision to assay the samples at the imprecise phase of late log growth may limit the repeatability of this experiment.
- The initial analysis of this data in terms of average termination efficiency fails to fully exploit the potential of flow cytometry data. A more sophisticated analysis could determine the distribution of single cell termination efficiencies.
- By assaying our constructs only at a single level of transcriptional initiation, we failed to explore the dependence of termination efficiency on the rate of PoPS input to the construct. We leave it to this later work to comment on the extent of agreement between our measured termination efficiencies.
Tables
Table 1: Terminator characterization constructs
| Construct | Terminator | Description |
|---|---|---|
| -- | -- | Negative Control: DH5a only |
| BBa_I7000 | -- | Positive Control: CFP only |
| BBa_I7001 | -- | Positive Control: YFP only |
| BBa_I7003 | -- | Postitive Control: CFP.YFP |
| BBa_I7010 | BBa_B0010 | T1 terminator |
| BBa_I7011 | BBa_B0011 | luxICDABEG operon terminator from V. fischeri |
| BBa_I7012 | BBa_B0012 | TE terminator from T7 |
| BBa_I7013 | BBa_B0013 | Variation on TE terminator from T7 |
| BBa_I7014 | BBa_B0014 | Double terminator: B0012.B0011 |
| BBa_I7015 | BBa_B0015 | Double terminator: B0010.B0012 |
| BBa_I7021 | BBa_B0021 | Reverse terminator of B0011 |
| BBa_I7022 | BBa_B0022 | Reverse terminator of B0012 |
| BBa_I7023 | BBa_B0023 | Reverse terminator of B0013 |
| BBa_I7025 | BBa_B0025 | Reverse terminator of B0015 |
Table 2: Terminator termination efficiencies (TE)
| Forward Terminator | Average Forward TE | Average Reverse TE | Reverse Terminator |
|---|---|---|---|
| BBa_B0011 | 41.9 % | 63.6 % | BBa_B0021 |
| BBa_B0012 | 30.9 % | -36.8 % | BBa_B0022 |
| BBa_B0013 | 60.0 % | -106.0 % | BBa_B0023 |
| BBa_B0014 | 60.4 % | * | BBa_B0024 |
| BBa_B0015 | 98.4 % | 29.5 % | BBa_B0025 |
Table 3: Terminator characterization flow data
| Construct | Terminator | Mean YFP (au) | Mean CFP (au) | TE |
|---|---|---|---|---|
| -- | Negative Control: DH5a only | 53 | 53 | n/a |
| BBa_I7000 | Positive Control: CFP only | 140 | 7149 | n/a |
| BBa_I7001 | Positive Control: YFP only | 13548 | 39 | n/a |
| BBa_I7003 | Postitive Control: CFP.YFP | 5483 | 1852 | 0.0 % |
| BBa_I7011 | BBa_B0011 | 4569 | 2658 | 41.9 % |
| BBa_I7012 | BBa_B0012 | 7821 | 3823 | 30.9 % |
| BBa_I7013 | BBa_B0013 | 5199 | 4388 | 60.0 % |
| BBa_I7014 | BBa_B0014 | 3975 | 3390 | 60.4 % |
| BBa_I7015 | BBa_B0015 | 324 | 6777 | 98.4 % |
| BBa_I7021 | BBa_B0021 | 3906 | 3627 | 63.6 % |
| BBa_I7022 | BBa_B0022 | 5312 | 1312 | -36.8 % |
| BBa_I7023 | BBa_B0023 | 9574 | 1570 | -106.0 % |
| BBa_I7025 | BBa_B0025 | 6945 | 3328 | 29.5 % |