Difference between revisions of "Part:BBa K792006"
Manugimenez (Talk | contribs) |
|||
| Line 4: | Line 4: | ||
'''TrpZipper''' is a small peptide that folds into a beta-hairpin secondary structure. The indole rings of the Trp form a hydrophobic core. The protein is water soluble and monomeric. | '''TrpZipper''' is a small peptide that folds into a beta-hairpin secondary structure. The indole rings of the Trp form a hydrophobic core. The protein is water soluble and monomeric. | ||
| − | [[image:TrpZipper2-structure.jpg]] | + | {| class="wikitable" width="170px" |
| − | Molecular structure of TrpZipper2 as determined by NMR (Cochran et al 2001). | + | |- |
| + | | [[image:TrpZipper2-structure.jpg | 170px]] | ||
| + | |- | ||
| + | |''Molecular structure of TrpZipper2 as determined by NMR (Cochran et al 2001).'' | ||
| + | |} | ||
<span class='h3bb'>Sequence and Features</span> | <span class='h3bb'>Sequence and Features</span> | ||
Revision as of 03:06, 29 October 2012
Tryptophan rich peptide (TrpZipper2)
TrpZipper is a small peptide that folds into a beta-hairpin secondary structure. The indole rings of the Trp form a hydrophobic core. The protein is water soluble and monomeric.
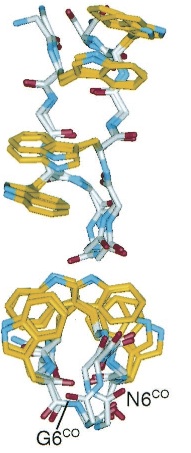
|
| Molecular structure of TrpZipper2 as determined by NMR (Cochran et al 2001). |
Sequence and Features
Assembly Compatibility:
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
