Difference between revisions of "Part:BBa K516132"
| Line 8: | Line 8: | ||
<br><br> | <br><br> | ||
| − | |||
===Usage and Biology=== | ===Usage and Biology=== | ||
| + | ==Shanghai_High_School 2019’s Characterisation== | ||
| + | ==BBa_K516132 Constitutive promoter [J23101] with mRFP, RBS B0032== | ||
| + | <p>The change in fluorescence intensity in the medium is related to the culture conditions, such as culture time, culture temperature, etc., in addition to the type of fluorescent protein. We studied this by experimenting with changes in culture time, culture temperature and fluorescence protein type.</p> | ||
| + | <p>1. We transformed the plasmid contanining BBa_K516132 and plasmid contanining BBa_K1073024 into bacterial competent cells separately, plated and cultured overnight at 37 °C. </p> | ||
| + | <p>2. On the evening of the next day, we picked single clone into a tube containing 5 ml LB which is called 0h. At the same time, we also inoculated competent DH5α strain into a tube containing 5 ml LB as a blank control. We culture bacterial cells at 37 ° C, 220 rpm. The sampling time point is 24h.</p> | ||
| + | <p>3. We took 100ul of the bacteria culture and measured the total fluorescence with a Thermo fluorescence microplate reader. We took 200ul of the ten-fold diluted bacterial solution and measured the OD600 with BioTek optical microplate reader. Total fluorescence is divided by OD600 to obtain fluorescence value per OD600.</p> | ||
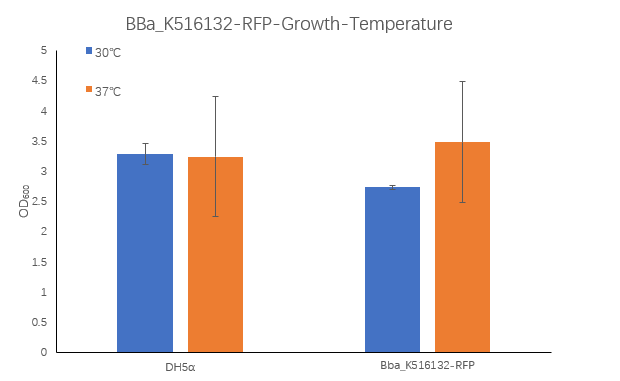
| + | [[Image:K516132-4.png|400px|Characterization of popular BioBrick RBSs]] | ||
| + | |||
| + | <strong>Figure1. BBa_K516132 containing strain OD600 at 30℃ and 37℃. </strong> | ||
| + | <p>The OD600 of blank control DH5α cells grown at 30 ° C and 37 ° C were 3.285 and 3.245, respectively, which is very close. The OD600 of the strain containing BBa_K516132 grown at 30 ° C was 2.735, which was lower than the OD600 3.485 at 37 ° C.</p> | ||
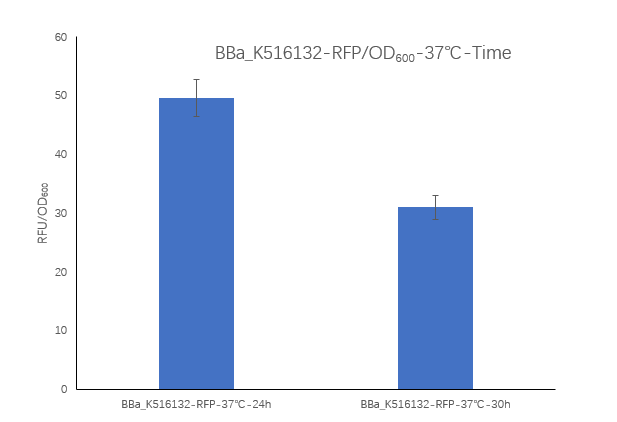
| + | [[Image:K516132-2.png|400px|Characterization of popular BioBrick RBSs]] | ||
| + | |||
| + | <strong>Figure2. Ba_K516132 containing strain Fluorescence per OD600 at 24h and 30h. </strong> | ||
| + | <p>We measured the RFU/OD600 of the strain at 24h and 30h, respectively. The RFU/OD600 at 24h is 49.57 ,which is higher than the RFU/OD600 31.04 at 30h. </p> | ||
| + | |||
| + | [[Image:K516132-2.png|400px|Characterization of popular BioBrick RBSs]] | ||
| + | |||
| + | <strong>Figure3. BBa_K516132 containing strain Fluorescence per OD600 at 30℃ and 37℃. </strong> | ||
| + | <p> We measured the RFU/OD600 of the strain at 30℃and 37℃, respectively. The DH5α RFU/OD600 at 24h and 30h is close to zero. The Bba_K516132 containing strain </p> | ||
| + | |||
| + | <p>RFU/OD600 is 45.26 at 37℃,which is higher than 3.74 at 30℃.</p> | ||
| + | |||
| + | <p>To sum up: the growth OD600 of the BBa_K516132-RFP strain is similar to the control DH5α, and this strain should have reached a stable phase at 24 h. The BBa_K516132-RFP strain has a much higher fluorescence at 37 ° C than 30 ° C.</p> | ||
| + | <p>These results indicate that the BBa_K516132-RFP protein may be expressed more or more stably at 37 °C, which also means that it may be necessary to find a suitable culture temperature when expressing an unknown protein.</p> | ||
| + | |||
<!-- --> | <!-- --> | ||
Revision as of 07:48, 20 October 2019
Constitutive promoter [J23101] with mRFP, RBS B0032
Protein Generator regulated by BBa_J23101 constitutive promoter with BBa_B0032 medium efficiency RBS, mRFP Reporter Coding Region and a double Transcription Terminators.
BBa_J23101 is the reference standard promoter for the computation of RPUs.
This measurement device belongs to a set of parts useful to estimate the RBS efficiency (BBa_K516130; BBa_K516131).
Usage and Biology
Shanghai_High_School 2019’s Characterisation
BBa_K516132 Constitutive promoter [J23101] with mRFP, RBS B0032
The change in fluorescence intensity in the medium is related to the culture conditions, such as culture time, culture temperature, etc., in addition to the type of fluorescent protein. We studied this by experimenting with changes in culture time, culture temperature and fluorescence protein type.
1. We transformed the plasmid contanining BBa_K516132 and plasmid contanining BBa_K1073024 into bacterial competent cells separately, plated and cultured overnight at 37 °C.
2. On the evening of the next day, we picked single clone into a tube containing 5 ml LB which is called 0h. At the same time, we also inoculated competent DH5α strain into a tube containing 5 ml LB as a blank control. We culture bacterial cells at 37 ° C, 220 rpm. The sampling time point is 24h.
3. We took 100ul of the bacteria culture and measured the total fluorescence with a Thermo fluorescence microplate reader. We took 200ul of the ten-fold diluted bacterial solution and measured the OD600 with BioTek optical microplate reader. Total fluorescence is divided by OD600 to obtain fluorescence value per OD600.
Figure1. BBa_K516132 containing strain OD600 at 30℃ and 37℃.
The OD600 of blank control DH5α cells grown at 30 ° C and 37 ° C were 3.285 and 3.245, respectively, which is very close. The OD600 of the strain containing BBa_K516132 grown at 30 ° C was 2.735, which was lower than the OD600 3.485 at 37 ° C.
Figure2. Ba_K516132 containing strain Fluorescence per OD600 at 24h and 30h.
We measured the RFU/OD600 of the strain at 24h and 30h, respectively. The RFU/OD600 at 24h is 49.57 ,which is higher than the RFU/OD600 31.04 at 30h.
Figure3. BBa_K516132 containing strain Fluorescence per OD600 at 30℃ and 37℃.
We measured the RFU/OD600 of the strain at 30℃and 37℃, respectively. The DH5α RFU/OD600 at 24h and 30h is close to zero. The Bba_K516132 containing strain
RFU/OD600 is 45.26 at 37℃,which is higher than 3.74 at 30℃.
To sum up: the growth OD600 of the BBa_K516132-RFP strain is similar to the control DH5α, and this strain should have reached a stable phase at 24 h. The BBa_K516132-RFP strain has a much higher fluorescence at 37 ° C than 30 ° C.
These results indicate that the BBa_K516132-RFP protein may be expressed more or more stably at 37 °C, which also means that it may be necessary to find a suitable culture temperature when expressing an unknown protein.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 7
Illegal NheI site found at 30 - 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal AgeI site found at 617
Illegal AgeI site found at 729 - 1000COMPATIBLE WITH RFC[1000]