Difference between revisions of "Part:BBa K3264004"
| Line 3: | Line 3: | ||
<partinfo>BBa_K3264004 short</partinfo> | <partinfo>BBa_K3264004 short</partinfo> | ||
| − | + | NT-W-CT is the recombinant spider silk protein (spidroin) that can spin into artificial spider silk fiber. This part is in the part collection where we provide improved recombinant chromoproteins and spidroins based on the functions of different regions of naturally occurring spidroin. By carrying out different mixing of the proteins of different combinations, we can produce artificially spun silk of different colors and properties. | |
| − | + | ||
| + | The part collection includes: | ||
| + | Parts that are different kinds of spidroins for silk spining. | ||
| + | <partinfo>BBa_K3264000</partinfo> | ||
| + | <partinfo>BBa_K3264005</partinfo> | ||
| + | <partinfo>BBa_K3264007</partinfo> | ||
| + | <partinfo>BBa_K3264009</partinfo> | ||
| + | <partinfo>BBa_K3264018</partinfo>. | ||
| + | <partinfo>BBa_K3264019</partinfo>. | ||
| + | Parts that for combining with the spidroins to form color silks | ||
| + | <partinfo>BBa_K3264012</partinfo>. | ||
| + | <partinfo>BBa_K3264013</partinfo>. | ||
| + | <partinfo>BBa_K3264014</partinfo>. | ||
| + | <partinfo>BBa_K3264015</partinfo>. | ||
| + | <partinfo>BBa_K3264016</partinfo>. | ||
| + | <partinfo>BBa_K3264017</partinfo>. | ||
| + | Part for adding more extensive repetitive region to the spidroin protein | ||
| + | <partinfo>BBa_K3264011</partinfo>. | ||
| + | |||
| + | Our part collection can help and inspire future teams to further design recombinant spider silks of more variety and give silks diversified color and property to fulfill the collection. | ||
| + | ate spidorins (MiSps). | ||
| − | |||
===Usage and Biology=== | ===Usage and Biology=== | ||
| + | A wide range of biomedical application of spider silk is occurring due to its features of high stress and strain tolerance. The current approach is to produce recombinant spidroins (silk proteins) from other chassis and spin them into silk. Though there are seven types of natural spider silks consist of their different spidroins, the common three regions of spidroins are basically the same: two non-repetitive terminal domains, and an extensive repetitive region that determines the type of the spidorin and its mechanical properties. | ||
| + | We aimed to synthesize the spidroin with the repeat modules from Argiope trifasciata (AcSp1) as our repetitive region, and we refer to the spidroin formed as NT-W-CT where NT from Euprosthenops australis and CT from Araneus ventricosus are respectively the N terminal domain that is monomeric and highly soluble in neutral pH (this feature provided the solubility of the entire spidroin) and C terminal domain. When pH is lowered from 7 to 5.5, NT can form stable dimers when pH decreases in the spinning duct, and the CT gets disrupted and unfold to turn in the beta-sheet amyloid-like fibrils. It is hypothesized that structural changes of CT precipitates the change of the repetitive region (W in this case) to beta-sheet conformation. In our experiments, NT-W-CT and NT-3W-CT can also uniformly form well distributed silk in 100% isopropanol. | ||
| + | We expect the final artificially spun fiber from the NTWCT spidroin to have high level of mechanical performance that is qualified to be applied to varieties of biomedical needs. | ||
| + | |||
| + | ==Reference== | ||
| + | [1] Zhou, Yizhong, et al. “Production and Properties of Triple Chimeric Spidroins.” Biomacromolecules, vol. 19, no. 7, 2018, pp. 2825–2833., doi:10.1021/acs.biomac.8b00402. | ||
| + | |||
| + | [2] Andersson, Marlene, et al. “Biomimetic Spinning of Artificial Spider Silk from a Chimeric Minispidroin.” /Nature Chemical Biology/, vol. 13, no. 3, Sept. 2017, pp. 262–264., doi:10.1038/nchembio.2269. | ||
| + | |||
| + | ==Characterization== | ||
| + | We aimed to use a synthetic biology approach to combine standardized DNA part NT-W-CT, and transform constructs to metabolically engineered ''E.coli'' for bioproduction. | ||
| + | |||
| + | ===Purification and SDS PAGE=== | ||
| + | [[File: ntwctfig1new.png|center|650px]] | ||
| + | [[File: ntwctfig1.png|center|650px]] | ||
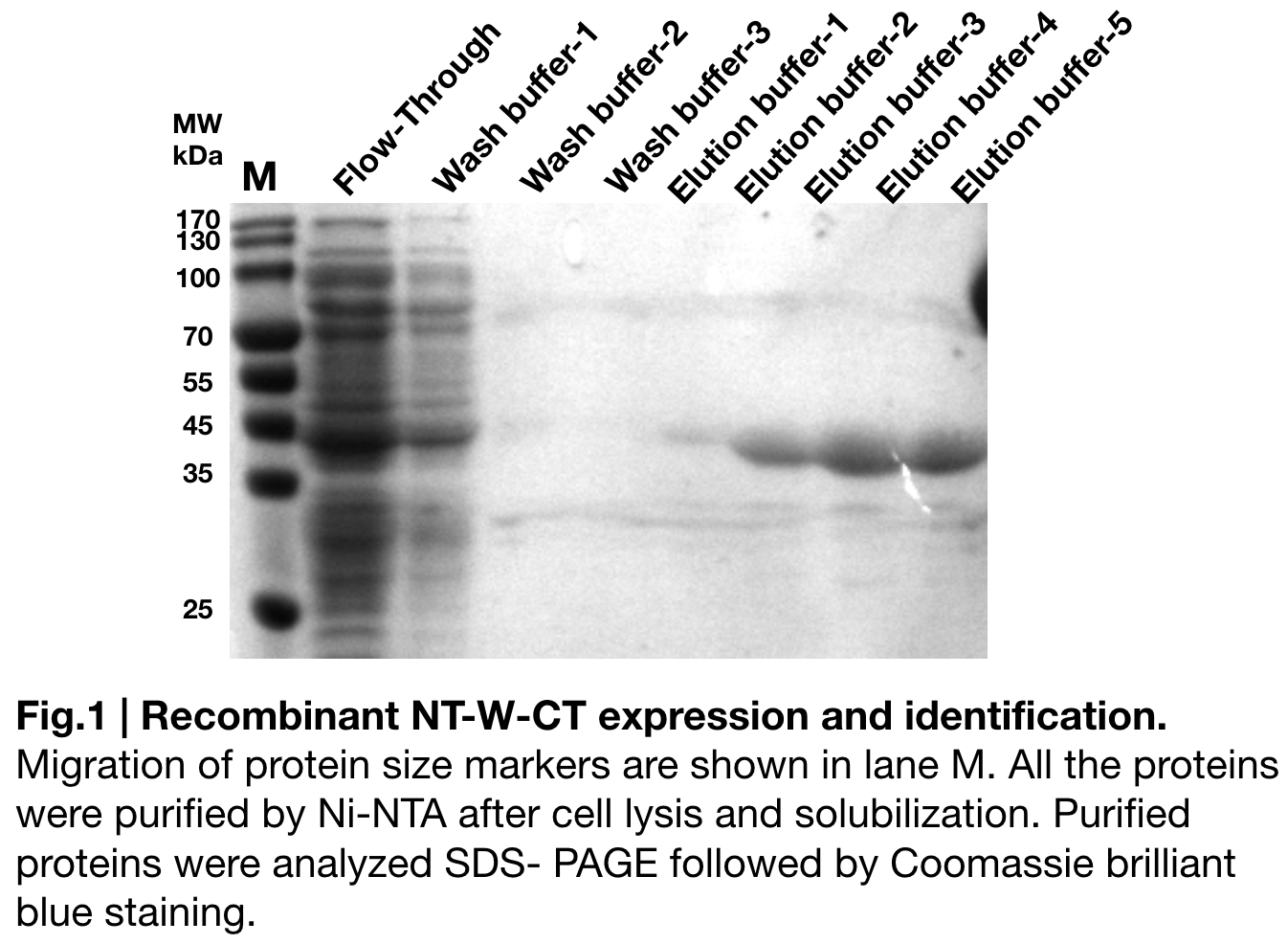
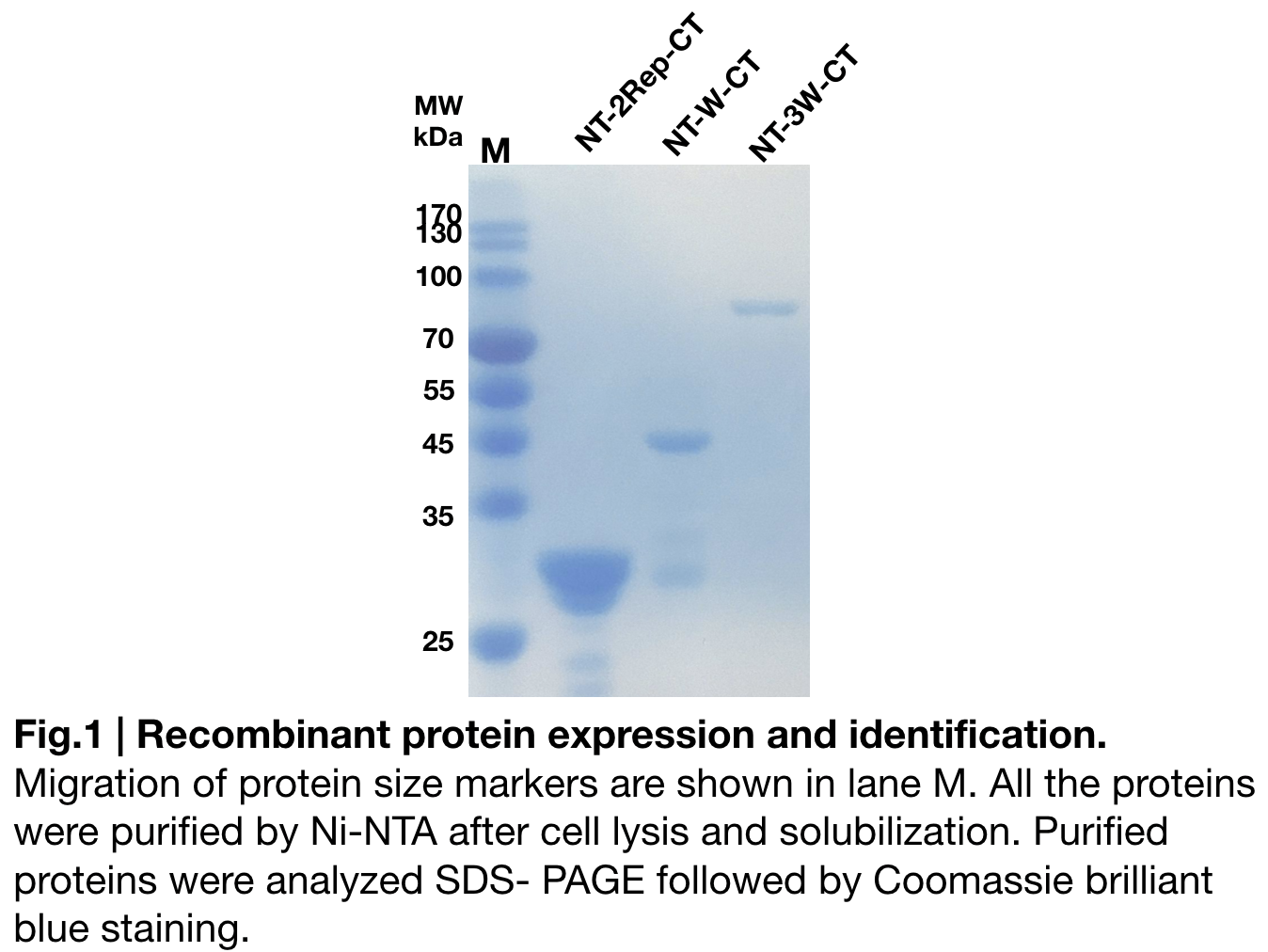
| + | In order to detect whether NT-W-CT protein expression was induced by adding isopropylthiogalactoside (final concentration 0.3mM), we used SDS-page(10%) to determine the presence of target protein. Target protein, NT-W-CT after purification by Ni-NTA method was also compared to NT-2Rep-CT and NT-3W-CT. Results showing the size of NT-2Rep-CT, NT-W-CT(45 kDa), and NT-3W-CT(80kDa) are in an increasing trend '''(Fig.2)''', this result is consistent with the previous research and the increase between NT-W-CT and NT-3W-CT shows us that extensive repetitive regions of W are added successfully and well induced. | ||
| + | |||
| + | |||
| + | |||
| + | ===Fibers spinning=== | ||
| + | [[File: ntwctfig2.png|center|650px]] | ||
| + | Uniformly distributed, silk can be formed by both NT-W-CT spidroin NT-3W-CT when we first spin it into 100% isopropanol. However, due to the easily self-assemble property of NT-W-CT protein, we were unable to artificially spin its mix with NT-W-CT spidroin into continuous silk, become the syringe head was stuck immediately after the dose of mixture enters the syringe and NT-W-CT starts to self-assemble and subsidies to the needle head. | ||
| + | |||
| + | Even in the dilute solution of our purified NT-W-CT protein that had been put in the 4C storage for 24 hours, we can obviously see the fiber like a self-assembling structure forming '''(Fig.2)'''. | ||
| + | |||
| + | [[File: ntwctfig3.png|center|650px]] | ||
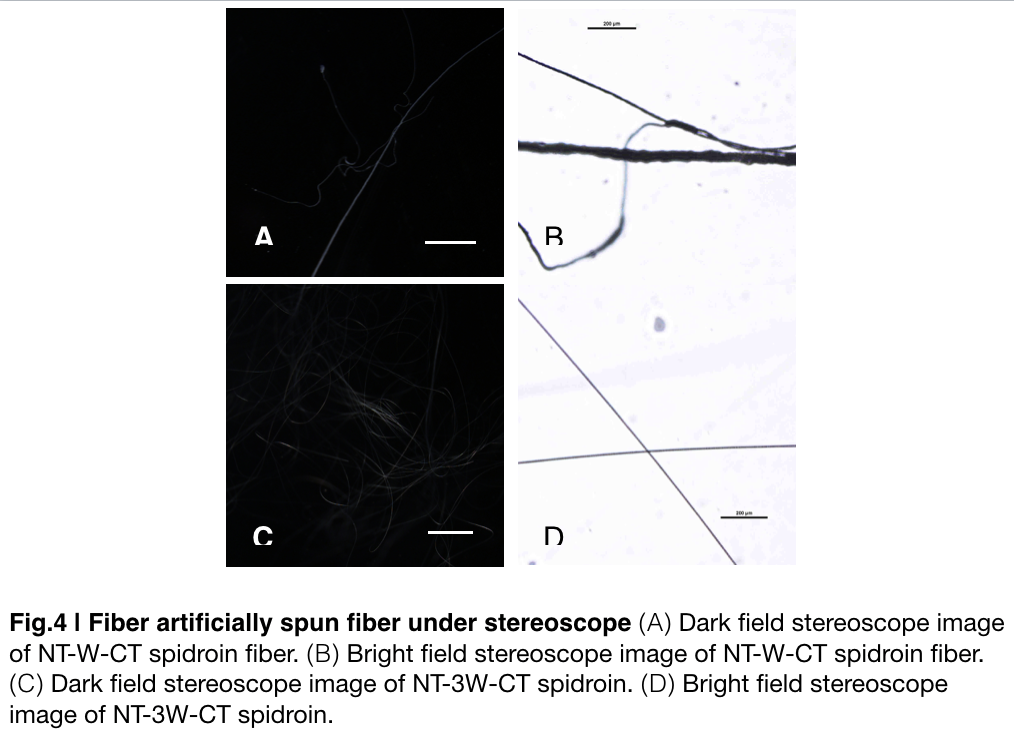
| + | Aiming to verify the distribution uniformity of NTWCT and NT3WCT after spinning into fiber. We randomly selected the cross section in our continuous silks to scan with stereocope'''(Fig.4)'''. It is clearly shown that the fiber circularity is of the silk spun by NT3WCT qualified as an uniformly distributed silk, however, silk formed by NTWCT has a variance in their diameter along the silk. The diameter of silk spun by NT-3W-CT (7.9um) is visually smaller than the average diameter of the silk spun by NT-W-CT. | ||
| + | |||
<!-- --> | <!-- --> | ||
Revision as of 22:41, 21 October 2019
W
NT-W-CT is the recombinant spider silk protein (spidroin) that can spin into artificial spider silk fiber. This part is in the part collection where we provide improved recombinant chromoproteins and spidroins based on the functions of different regions of naturally occurring spidroin. By carrying out different mixing of the proteins of different combinations, we can produce artificially spun silk of different colors and properties.
The part collection includes: Parts that are different kinds of spidroins for silk spining. BBa_K3264000 BBa_K3264005 BBa_K3264007 BBa_K3264009 BBa_K3264018. BBa_K3264019. Parts that for combining with the spidroins to form color silks BBa_K3264012. BBa_K3264013. BBa_K3264014. BBa_K3264015. BBa_K3264016. BBa_K3264017. Part for adding more extensive repetitive region to the spidroin protein BBa_K3264011.
Our part collection can help and inspire future teams to further design recombinant spider silks of more variety and give silks diversified color and property to fulfill the collection. ate spidorins (MiSps).
Usage and Biology
A wide range of biomedical application of spider silk is occurring due to its features of high stress and strain tolerance. The current approach is to produce recombinant spidroins (silk proteins) from other chassis and spin them into silk. Though there are seven types of natural spider silks consist of their different spidroins, the common three regions of spidroins are basically the same: two non-repetitive terminal domains, and an extensive repetitive region that determines the type of the spidorin and its mechanical properties. We aimed to synthesize the spidroin with the repeat modules from Argiope trifasciata (AcSp1) as our repetitive region, and we refer to the spidroin formed as NT-W-CT where NT from Euprosthenops australis and CT from Araneus ventricosus are respectively the N terminal domain that is monomeric and highly soluble in neutral pH (this feature provided the solubility of the entire spidroin) and C terminal domain. When pH is lowered from 7 to 5.5, NT can form stable dimers when pH decreases in the spinning duct, and the CT gets disrupted and unfold to turn in the beta-sheet amyloid-like fibrils. It is hypothesized that structural changes of CT precipitates the change of the repetitive region (W in this case) to beta-sheet conformation. In our experiments, NT-W-CT and NT-3W-CT can also uniformly form well distributed silk in 100% isopropanol. We expect the final artificially spun fiber from the NTWCT spidroin to have high level of mechanical performance that is qualified to be applied to varieties of biomedical needs.
Reference
[1] Zhou, Yizhong, et al. “Production and Properties of Triple Chimeric Spidroins.” Biomacromolecules, vol. 19, no. 7, 2018, pp. 2825–2833., doi:10.1021/acs.biomac.8b00402.
[2] Andersson, Marlene, et al. “Biomimetic Spinning of Artificial Spider Silk from a Chimeric Minispidroin.” /Nature Chemical Biology/, vol. 13, no. 3, Sept. 2017, pp. 262–264., doi:10.1038/nchembio.2269.
Characterization
We aimed to use a synthetic biology approach to combine standardized DNA part NT-W-CT, and transform constructs to metabolically engineered E.coli for bioproduction.
Purification and SDS PAGE
In order to detect whether NT-W-CT protein expression was induced by adding isopropylthiogalactoside (final concentration 0.3mM), we used SDS-page(10%) to determine the presence of target protein. Target protein, NT-W-CT after purification by Ni-NTA method was also compared to NT-2Rep-CT and NT-3W-CT. Results showing the size of NT-2Rep-CT, NT-W-CT(45 kDa), and NT-3W-CT(80kDa) are in an increasing trend (Fig.2), this result is consistent with the previous research and the increase between NT-W-CT and NT-3W-CT shows us that extensive repetitive regions of W are added successfully and well induced.
Fibers spinning
Uniformly distributed, silk can be formed by both NT-W-CT spidroin NT-3W-CT when we first spin it into 100% isopropanol. However, due to the easily self-assemble property of NT-W-CT protein, we were unable to artificially spin its mix with NT-W-CT spidroin into continuous silk, become the syringe head was stuck immediately after the dose of mixture enters the syringe and NT-W-CT starts to self-assemble and subsidies to the needle head.
Even in the dilute solution of our purified NT-W-CT protein that had been put in the 4C storage for 24 hours, we can obviously see the fiber like a self-assembling structure forming (Fig.2).
Aiming to verify the distribution uniformity of NTWCT and NT3WCT after spinning into fiber. We randomly selected the cross section in our continuous silks to scan with stereocope(Fig.4). It is clearly shown that the fiber circularity is of the silk spun by NT3WCT qualified as an uniformly distributed silk, however, silk formed by NTWCT has a variance in their diameter along the silk. The diameter of silk spun by NT-3W-CT (7.9um) is visually smaller than the average diameter of the silk spun by NT-W-CT.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal AgeI site found at 31
Illegal AgeI site found at 532 - 1000COMPATIBLE WITH RFC[1000]