Difference between revisions of "Part:BBa K3136029"
| Line 5: | Line 5: | ||
It is a composite part with promoter, RBS, conding sequence of CRISPR-Cas12a from Francisella novicida U112, and terminator. The sequence is coden optimized. We constuct the part as a coding sequence in typeIIS standard plasmid. | It is a composite part with promoter, RBS, conding sequence of CRISPR-Cas12a from Francisella novicida U112, and terminator. The sequence is coden optimized. We constuct the part as a coding sequence in typeIIS standard plasmid. | ||
| − | |||
===Usage and Biology=== | ===Usage and Biology=== | ||
| + | ===BBa_K3136029 J23100-B0034-FnCas12a-ter-typeIIS=== | ||
| + | |||
| + | ==Methods:== | ||
| + | <p>1. Put the EP tube containing purified FnCas12a protein at 99℃ water for 15 minutes.</p> | ||
| + | <p>2. After heating, centrifuge the tubes for 5min.</p> | ||
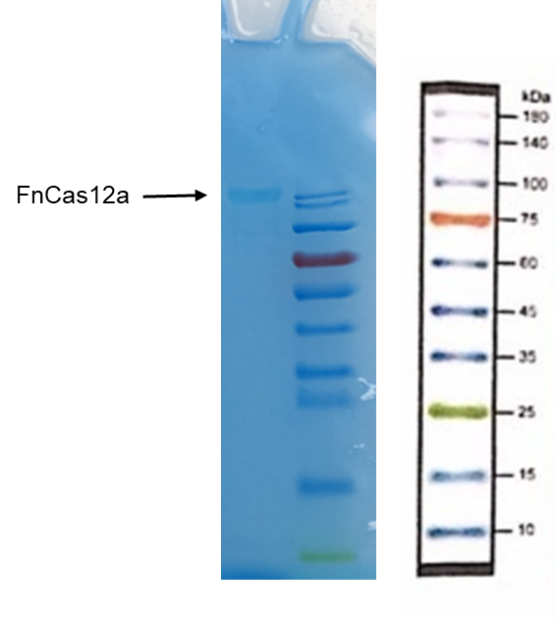
| + | <p>3. Take and install the gel, add the electrophoresis buffer to the sample hole, and check if there is any leakage. Add marker, to the first and last sample holes, add FnCas12a to the other sample holes, respectively.</p> | ||
| + | <p>7. Connect the electrophoresis device to the power supply. Connect the positive electrode to the tank and the negative electrode to the slot for electrophoresis. The voltage is adjusted to 160V. </p> | ||
| + | <p>8. Turn off the power supply and disconnect the electrode until the bromophenol blue reaches the bottom of the release adhesive. Remove the glass sheet from the electrophoresis device and then remove the gel.</p> | ||
| + | <p>9. Soak the gel in Coomassie brilliant blue dye and dye it in a horizontal shaking bed for 15 min.</p> | ||
| + | ===Observe the protein bands=== | ||
| + | [[Image:k3298010-1.png|400px|Characterization of popular BioBrick RBSs]] | ||
| + | ===Cas12a detection method:=== | ||
| + | |||
| + | <strong>Materials:</strong> | ||
| + | <p>Water. 14.5 μL</p> | ||
| + | <p>Buffer. 2 μL</p> | ||
| + | <p>Enzyme (Cas12a). 0.5 μL</p> | ||
| + | <p>Template. 0.5 μL</p> | ||
| + | <p>(From solution we made after LAMP)</p> | ||
| + | <p>Detector. 2 μL</p> | ||
| + | <p>crRNA. 0.5 μL</p> | ||
| + | <p>(Note: the Deteror is sequence of ssDNA HEX-N12-BHQ1 or FAM-N12-BHQ1 or FITC-T14-Biotin)</p> | ||
| + | ===Procedure:=== | ||
| + | <p>1. Add all the materials together:</p> | ||
| + | <p>• Firstly, use pipette to straw 14.5 μL pure water from the pure water bottle to one tube.</p> | ||
| + | <p>• Secondly, use pipette to straw 2 μL buffer to the previous tube.</p> | ||
| + | <p>• Thirdly, use pipette to straw 0.5 μL enzyme to the previous tube.</p> | ||
| + | <p>• Next, use pipette to straw 0.5 μL template to the previous tube.</p> | ||
| + | <p>• Then, add 0.5 μL crRNA to the previous tube.</p> | ||
| + | <p>• Finally, use pipette to straw 2 μL detector to the previous tube.</p> | ||
| + | <p>2. Straw 1 μL of the mixed solution in the tubes to the 96-well Plate.</p> | ||
| + | <p>3. Detect the solution using fluorescent detector machine to check the fluorescence when they react 10min.</p> | ||
| + | |||
| + | ==Results:== | ||
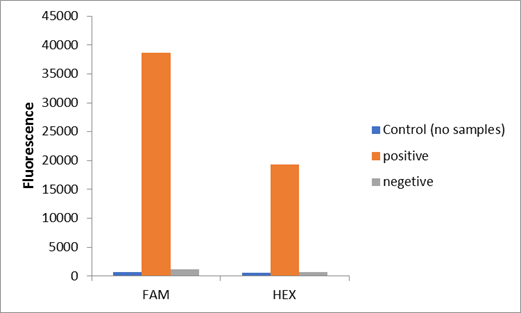
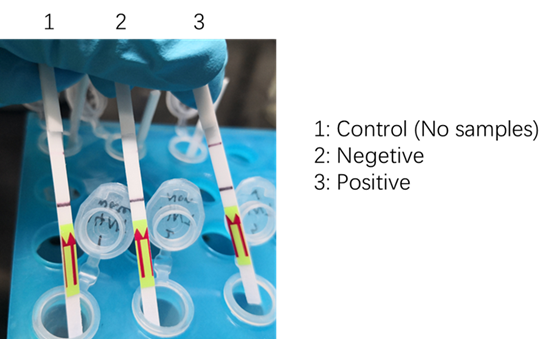
| + | <p>We used two types of fluorescent probe (FAM and HEX) to check the fluorescence of FnCas12a reaction system at 10min as shown in Fig1. Linked to the control and negative samples, the positive sample has a high fluorescence. It means that FnCas12a slices the fluorescent probe in the reaction system and the cracked fluorescent probe produce fluorescence. we also checked the fluorescence of FnCas12a reaction system at 10min by lateral flow using briDetect (Milenia Biotec GmbH) (Fig2).</p> | ||
| + | [[Image:k3298010-fig1.png|400px|center|Characterization of popular BioBrick RBSs]] | ||
| + | <center>Fig1. The fluorescence of FnCas12a reaction system at 10min</center> | ||
| + | [[Image:k3298010-fig2.png|400px|center|Characterization of popular BioBrick RBSs]] | ||
| + | <center>Fig2. The fluorescence of FnCas12a reaction system by briDetect</center> | ||
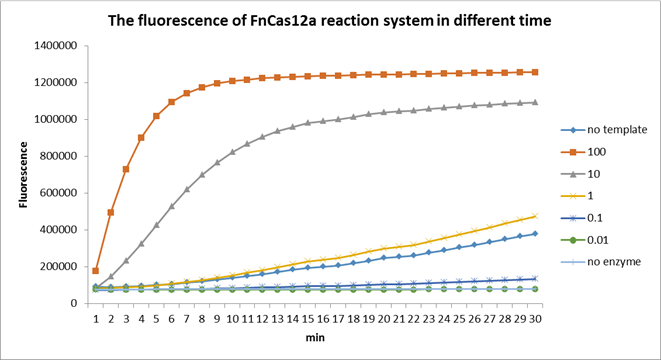
| + | <p>Using fluorescent detector machine, we can detect the fluorescence of FnCas12a reaction system at different time by setting up program properly in the machine. We detect the fluorescence under different concentration FnCas12a (form 0.01 nM to 100 nM) and different time(Fig3). With the time growth, the fluorescence gradually raised until a certain time. Higher concentration FnCas12a had higher fluorescence and the fluorescence increased quickly.</p> | ||
| + | [[Image:k3298010-fig3.png|400px|center|Characterization of popular BioBrick RBSs]] | ||
| + | <center>Fig3. The fluorescence of FnCas12a reaction system in different time. | ||
| + | |||
| + | </center> | ||
| + | |||
| + | ==Reference== | ||
| + | <p>Li S Y, Cheng Q X, Liu J K, et al. CRISPR-Cas12a has both cis-and trans-cleavage activities on single-stranded DNA[J]. Cell research, 2018, 28(4): 491.</p> | ||
| + | |||
<!-- --> | <!-- --> | ||
Latest revision as of 14:55, 19 October 2019
J23100-B0034-FnCas12a-ter-typeIIS
It is a composite part with promoter, RBS, conding sequence of CRISPR-Cas12a from Francisella novicida U112, and terminator. The sequence is coden optimized. We constuct the part as a coding sequence in typeIIS standard plasmid.
Usage and Biology
BBa_K3136029 J23100-B0034-FnCas12a-ter-typeIIS
Methods:
1. Put the EP tube containing purified FnCas12a protein at 99℃ water for 15 minutes.
2. After heating, centrifuge the tubes for 5min.
3. Take and install the gel, add the electrophoresis buffer to the sample hole, and check if there is any leakage. Add marker, to the first and last sample holes, add FnCas12a to the other sample holes, respectively.
7. Connect the electrophoresis device to the power supply. Connect the positive electrode to the tank and the negative electrode to the slot for electrophoresis. The voltage is adjusted to 160V.
8. Turn off the power supply and disconnect the electrode until the bromophenol blue reaches the bottom of the release adhesive. Remove the glass sheet from the electrophoresis device and then remove the gel.
9. Soak the gel in Coomassie brilliant blue dye and dye it in a horizontal shaking bed for 15 min.
Observe the protein bands
Cas12a detection method:
Materials:
Water. 14.5 μL
Buffer. 2 μL
Enzyme (Cas12a). 0.5 μL
Template. 0.5 μL
(From solution we made after LAMP)
Detector. 2 μL
crRNA. 0.5 μL
(Note: the Deteror is sequence of ssDNA HEX-N12-BHQ1 or FAM-N12-BHQ1 or FITC-T14-Biotin)
Procedure:
1. Add all the materials together:
• Firstly, use pipette to straw 14.5 μL pure water from the pure water bottle to one tube.
• Secondly, use pipette to straw 2 μL buffer to the previous tube.
• Thirdly, use pipette to straw 0.5 μL enzyme to the previous tube.
• Next, use pipette to straw 0.5 μL template to the previous tube.
• Then, add 0.5 μL crRNA to the previous tube.
• Finally, use pipette to straw 2 μL detector to the previous tube.
2. Straw 1 μL of the mixed solution in the tubes to the 96-well Plate.
3. Detect the solution using fluorescent detector machine to check the fluorescence when they react 10min.
Results:
We used two types of fluorescent probe (FAM and HEX) to check the fluorescence of FnCas12a reaction system at 10min as shown in Fig1. Linked to the control and negative samples, the positive sample has a high fluorescence. It means that FnCas12a slices the fluorescent probe in the reaction system and the cracked fluorescent probe produce fluorescence. we also checked the fluorescence of FnCas12a reaction system at 10min by lateral flow using briDetect (Milenia Biotec GmbH) (Fig2).
Using fluorescent detector machine, we can detect the fluorescence of FnCas12a reaction system at different time by setting up program properly in the machine. We detect the fluorescence under different concentration FnCas12a (form 0.01 nM to 100 nM) and different time(Fig3). With the time growth, the fluorescence gradually raised until a certain time. Higher concentration FnCas12a had higher fluorescence and the fluorescence increased quickly.
Reference
Li S Y, Cheng Q X, Liu J K, et al. CRISPR-Cas12a has both cis-and trans-cleavage activities on single-stranded DNA[J]. Cell research, 2018, 28(4): 491.
Sequence and Features
- 10INCOMPATIBLE WITH RFC[10]Illegal PstI site found at 371
Illegal PstI site found at 425
Illegal PstI site found at 1688
Illegal PstI site found at 2594
Illegal PstI site found at 3788 - 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 7
Illegal NheI site found at 30
Illegal PstI site found at 371
Illegal PstI site found at 425
Illegal PstI site found at 1688
Illegal PstI site found at 2594
Illegal PstI site found at 3788 - 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 1314
Illegal BglII site found at 1647
Illegal BglII site found at 1767
Illegal BglII site found at 2061
Illegal BglII site found at 2505
Illegal BglII site found at 3396
Illegal BglII site found at 3486 - 23INCOMPATIBLE WITH RFC[23]Illegal PstI site found at 371
Illegal PstI site found at 425
Illegal PstI site found at 1688
Illegal PstI site found at 2594
Illegal PstI site found at 3788 - 25INCOMPATIBLE WITH RFC[25]Illegal PstI site found at 371
Illegal PstI site found at 425
Illegal PstI site found at 1688
Illegal PstI site found at 2594
Illegal PstI site found at 3788
Illegal NgoMIV site found at 2541
Illegal NgoMIV site found at 3380
Illegal NgoMIV site found at 3591 - 1000COMPATIBLE WITH RFC[1000]