Difference between revisions of "Part:BBa K3114022"
| Line 49: | Line 49: | ||
<partinfo>BBa_K3114022 SequenceAndFeatures</partinfo> | <partinfo>BBa_K3114022 SequenceAndFeatures</partinfo> | ||
| + | |||
===References=== | ===References=== | ||
Revision as of 04:35, 21 October 2019
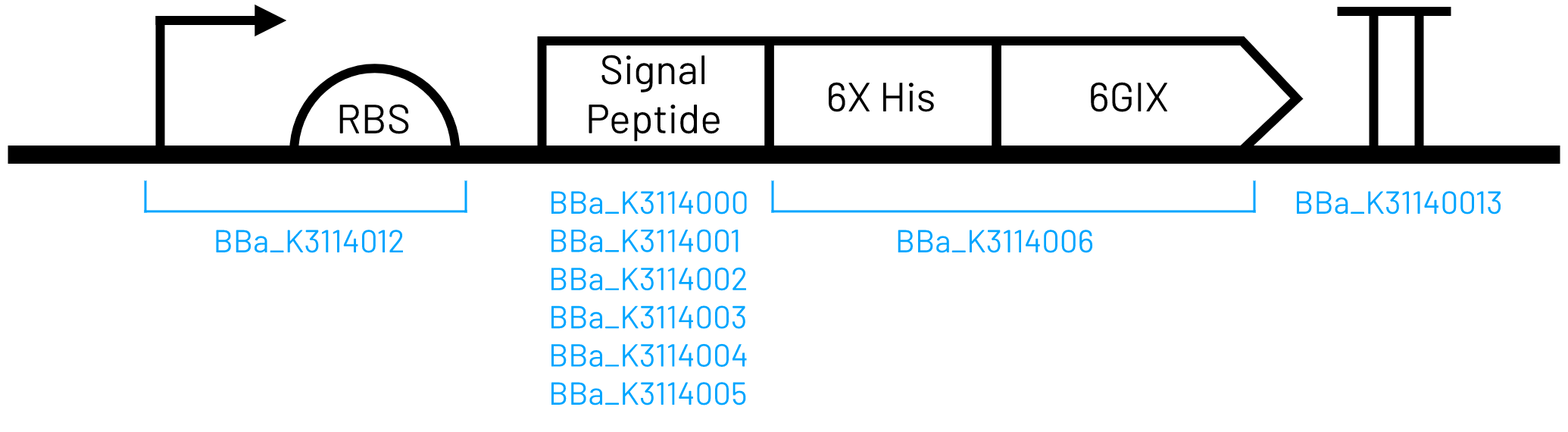
6xHis-tagged water-soluble chlorophyll binding protein (6GIX) circuit with no signal peptide
Usage and Biology
This part can be used for IPTG-inducible expression and secretion of the water-soluble chlorophyll binding protein 6GIX, which contains a 6xHis tag for purification. This circuit does not contain a signal peptide.
iGEM Calgary successfully created seven inducible genetic circuits for high-level production of 6GIX using various parts from our collection.
- DsbA signal peptide 6GIX circuit (BBa_K3114016)
- MalE signal peptide 6GIX circuit (BBa_K3114017)
- OmpA signal peptide 6GIX circuit (BBa_K3114018)
- PhoA signal peptide 6GIX circuit (BBa_K3114019)
- YcbK signal peptide 6GIX circuit (BBa_K3114020)
- TorA signal peptide 6GIX circuit (BBa_K3114021)
- 6GIX circuit with no signal peptide (BBa_K3114022)
6GIX is a 180-amino acid water-soluble chlorophyll binding protein (WSCP) which is hypothesized to play a role as a transient chlorophyll shuttle or to be involved in anti-photobleaching responses in Lepidium virginicum (Takahashi et al., 2013). 6GIX is capable of binding chlorophyll a and b, but it has been shown to have higher affinity for chlorophyll b (Bednarczyk, Takahashi, Satoh, & Noy, 2015; Palm et al., 2018).
6GIX exists as a homotetramer that is capable of binding four chlorophyll molecules (Bednarczyk, Takahashi, Satoh, & Noy, 2015). Chlorophyll is a hydrophobic pigment and is therefore soluble only in organic solvents. This part can be used for aqueous phase capture of chlorophyll using emulsions.
Design
When designing this circuit and the rest of our collection, we were interested in creating parts that could be used in Golden Gate assembly right out of the distribution kit without the need to first domesticate them in a Golden Gate entry vector. As such, the basic parts are not compatible with the iGEM Type IIS RFC[1000] assembly standard because we included the BsaI restriction site and MoClo standard fusion site in the part’s sequence.
This part was constructed using component parts listed below. It is iGEM Type IIS RFC[1000] compatible.
- T7 Promoter and strong RBS (BBa_K3114012)
- 6xHis-tagged 6GIX (BBa_K3114006)
- Double terminator (BBa_K3114013)
The circuit was designed for inducible, high-level expression and has been codon optimized for E. coli. A 6X Histidine affinity chromatography tag was added to the N-terminus of the 6GIX coding sequence for purification.
Characterization
We were able to purify 6GIX produced by this genetic construct using the 6xHis tag and Ni-NTA column chromatography. The SDS-PAGE gel below shows the protein in the whole cell lysate (WCL) and in different elution fractions following purification. Purification was conducted as per our protocol. The second elution fraction shows the strongest band. The empty destination vector (EVC) was used as a control.
For experimental characterization of 6GIX’s function, please see (BBa_K3114006).
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 654
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 179
Illegal AgeI site found at 122
Illegal AgeI site found at 438 - 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI site found at 1
Illegal BsaI.rc site found at 765
References
Bednarczyk, D., Takahashi, S., Satoh, H., & Noy, D. (2015). Assembly of water-soluble chlorophyll-binding proteins with native hydrophobic chlorophylls in water-in-oil emulsions. Biochimica et Biophysica Acta - Bioenergetics, 1847(3), 307–313. https://doi.org/10.1016/j.bbabio.2014.12.003
Palm, D. M., Agostini, A., Averesch, V., Girr, P., Werwie, M., Takahashi, S., … Paulsen, H. (2018). Chlorophyll a/b binding-specificity in water-soluble chlorophyll protein. Nature Plants, 4(11), 920–929. https://doi.org/10.1038/s41477-018-0273-z
Takahashi, S., Yanai, H., Oka-Takayama, Y., Zanma-Sohtome, A., Fujiyama, K., Uchida, A., … Satoh, H. (2013). Molecular cloning, characterization and analysis of the intracellular localization of a water-soluble chlorophyll-binding protein (WSCP) from Virginia pepperweed (Lepidium virginicum), a unique WSCP that preferentially binds chlorophyll b in vitro. Planta, 238(6), 1065–1080. https://doi.org/10.1007/s00425-013-1952-7
Weber, E., Engler, C., Gruetzner, R., Werner, S., & Marillonnet, S. (2011). A modular cloning system for standardized assembly of multigene constructs. PLoS ONE, 6(2). https://doi.org/10.1371/journal.pone.0016765