Difference between revisions of "Part:BBa K2586019"
| Line 1: | Line 1: | ||
| − | |||
__NOTOC__ | __NOTOC__ | ||
<partinfo>BBa_K2586019 short</partinfo> | <partinfo>BBa_K2586019 short</partinfo> | ||
| − | + | <html> | |
| + | <p align="justify"> | ||
| + | <br> | ||
This part encodes for an enzyme that n-acetylates the herbicide and antibiotic glyphosate. | This part encodes for an enzyme that n-acetylates the herbicide and antibiotic glyphosate. | ||
The GAT-enzyme is able to confer glyphosate resistance to organisms such as <i>Escherichia coli</i>, <i>Arabidopsis</i>, tobacco and maize. | The GAT-enzyme is able to confer glyphosate resistance to organisms such as <i>Escherichia coli</i>, <i>Arabidopsis</i>, tobacco and maize. | ||
The acetylation of glyphosate may provide a new and alternative strategy in glyphosate support on crop plants. | The acetylation of glyphosate may provide a new and alternative strategy in glyphosate support on crop plants. | ||
We integrated this part into soil bacterium <i>Bacillus subtilis</i> to enable the bacteria to inactivate glyphosate, which would otherwise be deadly to them. | We integrated this part into soil bacterium <i>Bacillus subtilis</i> to enable the bacteria to inactivate glyphosate, which would otherwise be deadly to them. | ||
| + | |||
| + | </html> | ||
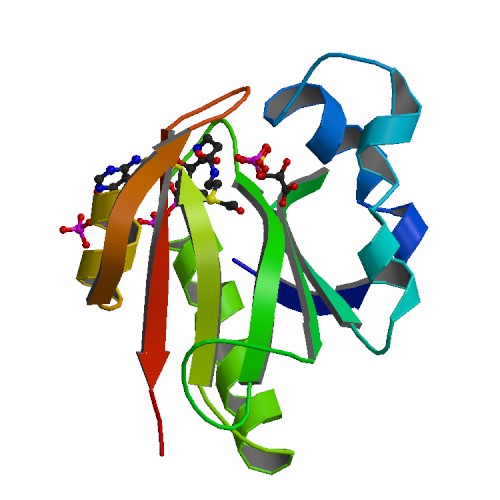
| + | [[File:T--goettingen--structure GAT.png|300px|right|thumb|<b>Protein structure of Glyphosate N-acetyltransferase bound to acetyl COA and 3-phosphoglycerate</b>]] | ||
| + | <html> | ||
| + | <br><br><br><br> | ||
| + | <style type="text/css"> | ||
| + | .tg {border-collapse:collapse;border-spacing:0;} | ||
| + | .tg td{font-family:Arial, sans-serif;font-size:14px;padding:0px 20px;border-style:solid;border-width:0px;overflow:hidden;word-break:normal;border-top-width:1px;border-bottom-width:1px;border-color:black;} | ||
| + | .tg th{font-family:Arial, sans-serif;font-size:14px;font-weight:normal;padding:0px 20px;border-style:solid;border-width:0px;overflow:hidden;word-break:normal;border-top-width:1px;border-bottom-width:1px;border-color:black;} | ||
| + | .tg .tg-4ovm{font-weight:bold;font-size:13px;border-color:#000000;text-align:left;vertical-align:top} | ||
| + | .tg .tg-9mbj{font-size:13px;border-color:#000000;text-align:left;vertical-align:top} | ||
| + | </style> | ||
| + | <table class="tg"> | ||
| + | <tr> | ||
| + | <td class="tg-4ovm">Molecular weight</td> | ||
| + | <td class="tg-9mbj">17 kDa</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td class="tg-4ovm">Protein length</td> | ||
| + | <td class="tg-9mbj">146 aa</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td class="tg-4ovm">Gene length</td> | ||
| + | <td class="tg-9mbj">483 bp</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td class="tg-4ovm">Function</td> | ||
| + | <td class="tg-9mbj">mediates glyphosate tolerance</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td class="tg-4ovm">Product</td> | ||
| + | <td class="tg-9mbj">N-acetyl glyphosate</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td class="tg-4ovm">Ideal pH</td> | ||
| + | <td class="tg-9mbj">6.8</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td class="tg-4ovm">Essential</td> | ||
| + | <td class="tg-9mbj">no</td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | |||
| + | </html> | ||
| Line 13: | Line 58: | ||
<!-- --> | <!-- --> | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
<span class='h3bb'>Sequence and Features</span> | <span class='h3bb'>Sequence and Features</span> | ||
<partinfo>BBa_K2586019 SequenceAndFeatures</partinfo> | <partinfo>BBa_K2586019 SequenceAndFeatures</partinfo> | ||
| Line 21: | Line 71: | ||
<partinfo>BBa_K2586019 parameters</partinfo> | <partinfo>BBa_K2586019 parameters</partinfo> | ||
<!-- --> | <!-- --> | ||
| + | |||
| + | ==Characterization== | ||
| + | ===<b>Engineering bacteria to disarm glyphosate</b>=== | ||
| + | <html> | ||
| + | <p align="justify"> | ||
| + | |||
| + | Many years ago it has been found that glyphosate can be inactivated by acetylation yielding N-acetylglyphosate (<a href="https://www.ncbi.nlm.nih.gov/pubmed/15155947"> Castle et al.,2004</a>). Because the acetylated form of glyphosate has low affinity for the active site of EPSP synthase, it is nonherbicidal. The glyphosate N-acetyltransferase GAT from the Gram-positive soil bacterium <i>Bacillus licheniformis</i> transfers the acetyl group from acetyl-CoA onto the amino group of the weedkiller (<a href="https://www.ncbi.nlm.nih.gov/pubmed/15155947"> Castle et al.,2004</a>).<br><br> Interestingly, <i>B. subtilis</i> possesses a putative glyphosate N-acetyltransferase, which is designated as YitI and shares 59% overall amino acid identity with GAT of B. licheniformis. However, the native GAT enzymes are very poor catalysts for N-acetylation of glyphosate (<a href="https://www.ncbi.nlm.nih.gov/pubmed/15155947"> Castle et al.,2004</a>). Moreover, despite extensive screening of biological amines, including amino acids, nucleotides and antibiotics, the physiological substrates for the native enzymes are unknown (Siehl et al., 2007 JBC). As an alternative strategy for glyphosate resistance involving enzymatic conversion of glyphosate to N-acetylglyphosate the GAT was also subjected to directed evolution for creating an enzyme with higher efficiency and increased specificity for the herbicide (<a href="https://www.ncbi.nlm.nih.gov/pubmed/15155947"> Castle et al.,2004</a>). When introduced into plants, optimized gat genes confer robust tolerance to glyphosate (<a href="https://www.ncbi.nlm.nih.gov/pubmed/15155947"> Castle et al.,2004</a>). Therefore, we expect that bacteria like <i>B. subtilis</i> expressing the optimized GAT should tolerate high amounts of glyphosate even though it is taken up from the environment via the high-affinity glyphosate transporter GltT (see above). To evaluate the potential of the GAT to allow <i>B. subtilis</i> in the presence of glyphosate concentrations that are toxic for the wild type bacteria, we wanted to express the gat gene in <i>B. subtilis</i> (Figure 1). | ||
| + | </p> | ||
| + | <br style="clear: both" /> | ||
| + | </html> | ||
| + | |||
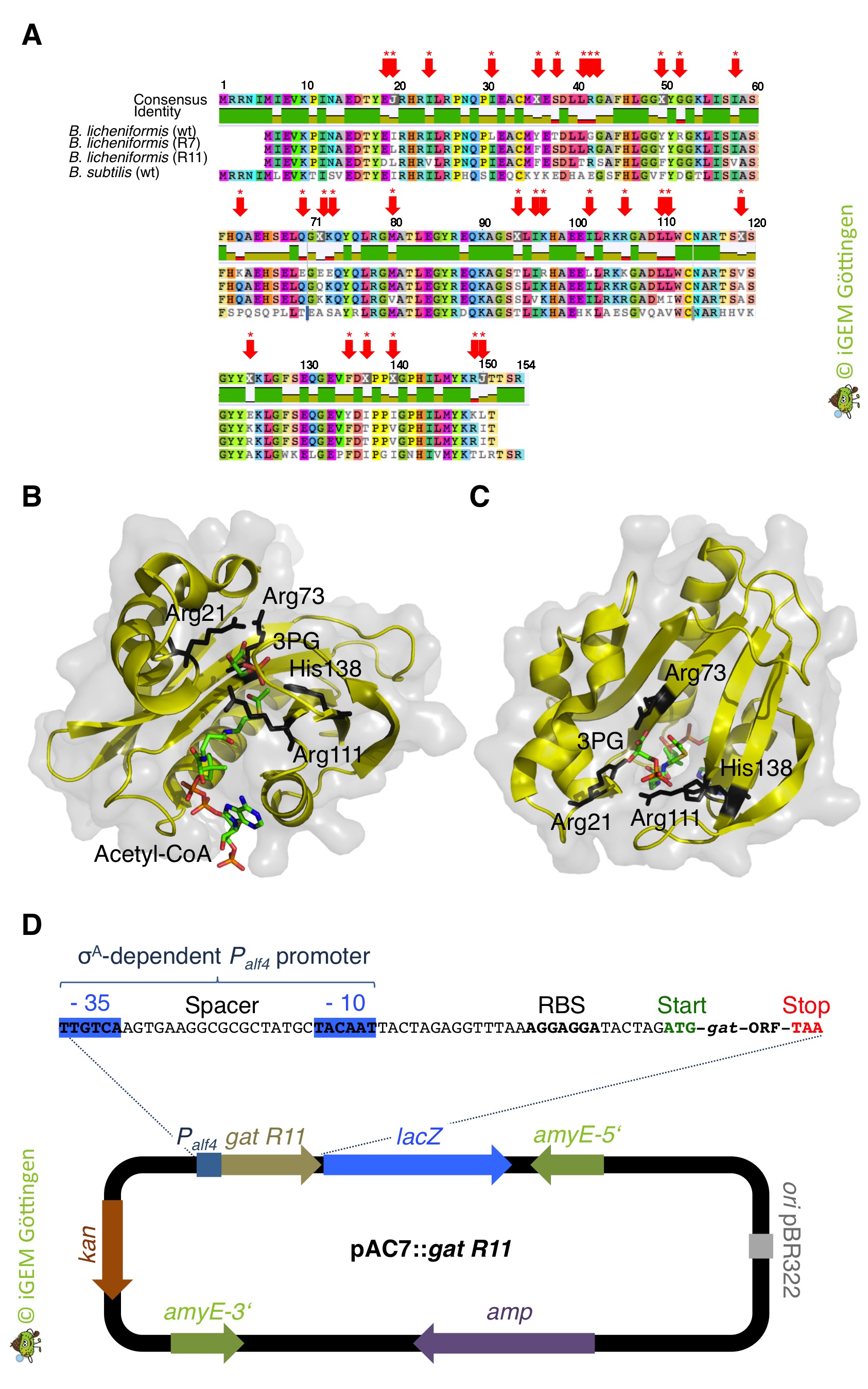
| + | [[File:T--goettingen--gat engineering.jpg|700px|center|thumb|'''Fig. 1.''' <b>A.</b> Sequence alignment showing the similarities of the wild type (wt) gat alleles from Bacillus licheniformis and <i>Bacillus subtilis</i> as well as the gat alleles R7 and R11 from B. licheniformis that were obtained by 11 rounds of gene shuffling. The red arrows indicate the 31 amino acid exchanges in the glyphosate N-acetyltransferase (Gat) variant R11. The optimized Gat variants shown an up to a 4,500-fold increase in catalytic efficiency (kcat/Km) relative to the native enzyme. The GenBank accession numbers for the sequences: B. licheniformis ST401 GAT, AX543338; R7 GAT, AY597417; R11 GAT, AY597418; <i>B. subtilis</i> YitI, CAA70664. B. Structure of the Gat R7 variant (PDBid: 2JDD) in ternary complex with acetyl-CoA and the competitive inhibitor 3-phosphoglycerate (3PG). The four active site residues (Arg-21, Arg-73, Arg-111, and His-138), which are labelled in black color, contribute to a positively charged substrate-binding site that is conserved throughout the GAT subfamily. C. Top view on the active site of Gat R7. D. Construction of a plasmid for the expression of the gat R11 variant in <i>B. subtilis</i> . Expression is driven by the constitutively active σA-dependent Palf4 promoter The plasmid pAC7::gat R11 integrates into the amyE locus of the <i>B. subtilis</i> chromosome in single copy. lacZ, beta-galactosidase; amyE-5' and amyE-3', fragments of the <i>B. subtilis amyE</i> for homologous recombination; <i>amp</i>, ampicillin resistance gene for the selection in <i>E. coli; kan</i>, kanamycin resistance gene for the selection in <i>B. subtilis</i> ; ori pBR322, origin of replication for <i>E. coli</i>. ]] | ||
| + | <html> | ||
| + | <hr> | ||
| + | </html> | ||
| + | |||
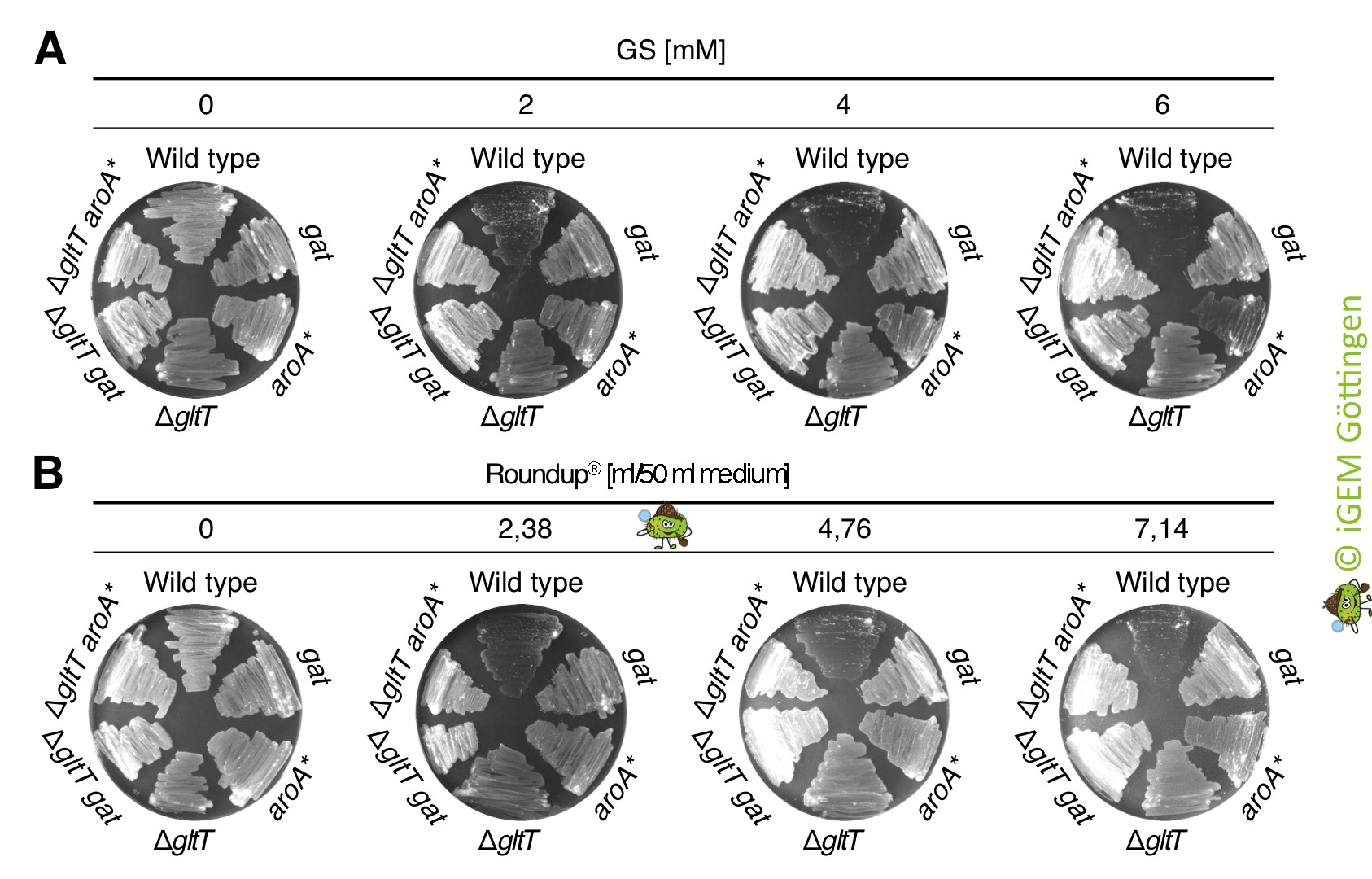
| + | [[File:T--goettingen--roundup aroA gat sp1.jpg|400px|thumb|'''Fig. 2.''' <b>A.</b> Growth of the wild type and ΔgltT mutant and of the isogenic strains overexpressing the <i>gat</i> R11 and <i>aroA*</i> genes on CS-Glc minimal medium in the presence of different amounts of glyphosate. <b>B.</b> Growth of the strains described in A on CS-Glc minimal medium in the presence of different amounts of Roundup® Alphee. ]] | ||
| + | |||
| + | ===<b>GAT confers tolerance to glyphosate</b>=== | ||
| + | <html> | ||
| + | <p align="justify"> | ||
| + | |||
| + | We ordered the gat gene as a g-Block from Integrated DNA Technologies, which was free of charge because the company supports the iGEM competition. Next, we amplified the gat gene by PCR using the g-Block as template DNA. An artificial promoter and a ribosome binding site was attached during the PCR (Figure 1). The promoter-gene fusion was cloned and introduced in single copy into the genomes of the <i>B. subtilis</i> wild type strain and the <i>gltT</i> mutant strain to further increase its glyphosate tolerance (Figure 2). To assess the potential of the GAT to disarm glyphosate in the different <i>B. subtilis</i> strains, we propagated the bacteria on CS-Glc minimal medium agar plates that were supplemented with different amounts of glyphosate. As shown in Figure 2, <b>the strains synthesizing the GAT tolerated significantly more glyphosate than the wild type strain</b>. Moreover, the inactivation of the gltT glyphosate transporter gene and the overexpression of the gat gene confers high-level glyphosate tolerance to the bacteria. Thus, when introduced into <i>B. subtilis</i> , the optimized gat gene confers indeed robust tolerance to glyphosate. To conclude, the engineered bacteria possess two interesting properties:<b> (i) <i>B. subtilis</i> promotes plant growth by protecting plants against pathogens and (ii), the bacteria take up glyphosate from the environment and inactivate the weedkiller!</b> | ||
| + | </p> | ||
| + | <br style="clear: both" /> | ||
| + | </html> | ||
| + | <html> | ||
| + | <hr> | ||
| + | </html> | ||
| + | |||
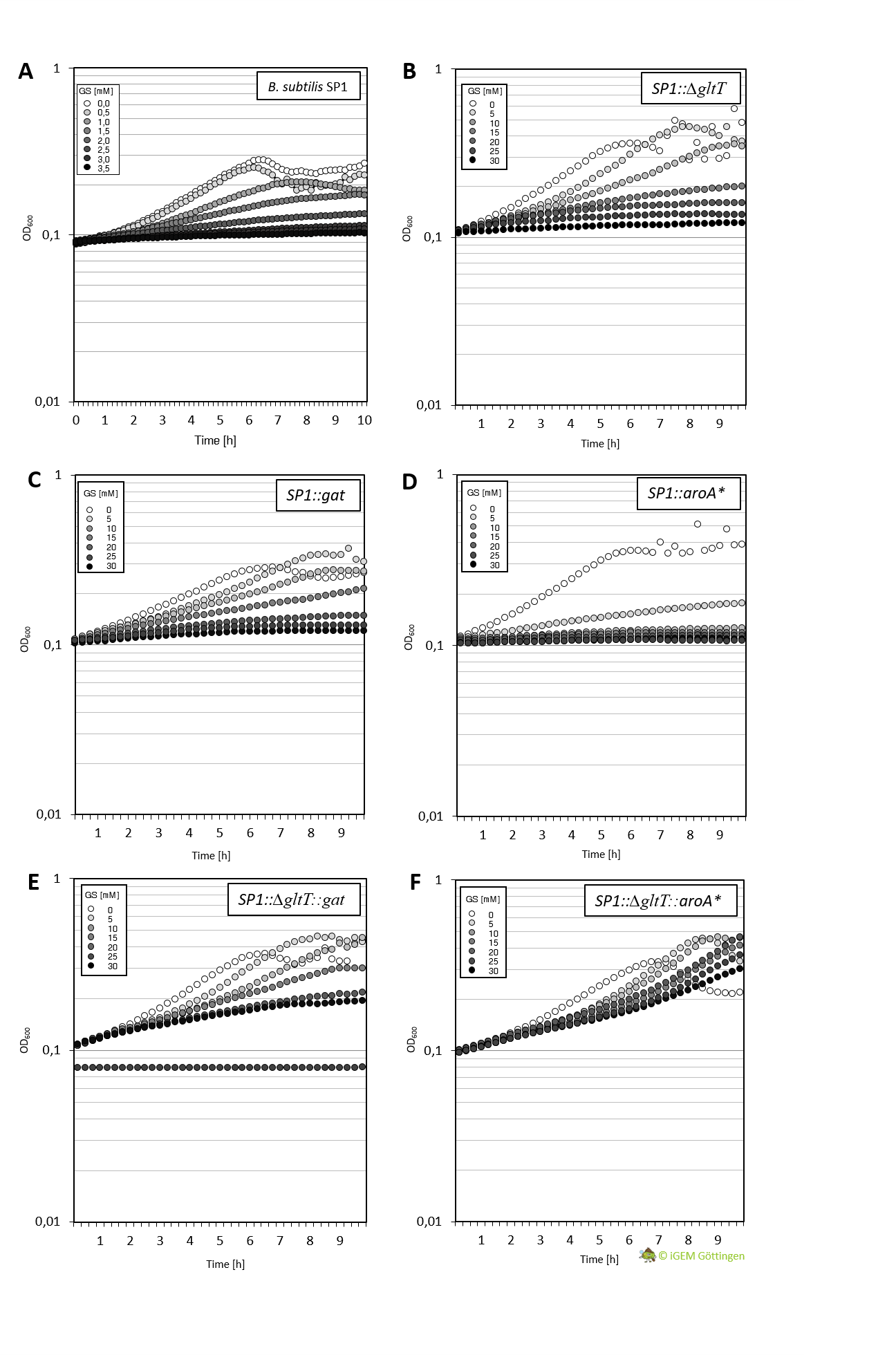
| + | [[File:T--Goettingen--growtharoa-gat.png|440px|thumb|'''Fig. 3.''' <b>A.</b> Growth of the B. subtilis wild type strain in presence of 0 - 3.5 mM Glyphosate. B. Growth of the ∆gltT B. subtilis strain with increasing glyphosate concentration (0-30 mM). C. Growth of the gat B. subtilis strain with increasing glyphosate concentration (0-30 mM. D.Growth of the aroA* B. subtilis strain with increasing glyphosate concentration (0-30 mM E. Growth of the aroA* B. subtilis strain with increasing glyphosate concentration (0-30 mM). Growth of the B. subtilis strain containing both ∆gltT and gat with increasing glyphosate concentration (0-30 mM). F. Growth of the B. subtilis strain containing both ∆gltT and aoA* with increasing glyphosate concentration (0-30 mM). Bacteria were cultivated in liquid Cs-Glu medium. ]] | ||
| + | |||
| + | ===<b>Characeterization of GAT conferred tolerance to glyphosate</b>=== | ||
| + | <html> | ||
| + | <p align="justify"> | ||
| + | |||
| + | Cultivation of the different strains in liquid media allows more detailed characterisation. We analysed growth of the bacteria in CS-Glu medium supplemented with increasing amounts of glyphosate, using a platereader. | ||
| + | In general, the analysis supports previous results. The strain containing <i>aroA*</i> confers only a slight increase in resistance in comparison to the WT strain and hence a significantly lower resistance in comparison the <i>∆gltT</i> strain (Figure 3 A, B and D ). However, for the strain combining <i>aroA*</i> and <i>∆gltT</i> a 9-fold higher glyphosate concentration is needed to reduce the growth rate by 50% (Figure 3 F. Figure Y ). <br><br> | ||
| + | Further characterisation of the Gat enzyme supports previous results and underline significant increased resistance in both strains conferring gat or a combination of <i>gat</i> and <i>∆gltT</i> (Figure 3 C and E, Figure 4). | ||
| + | </p> | ||
| + | </html> | ||
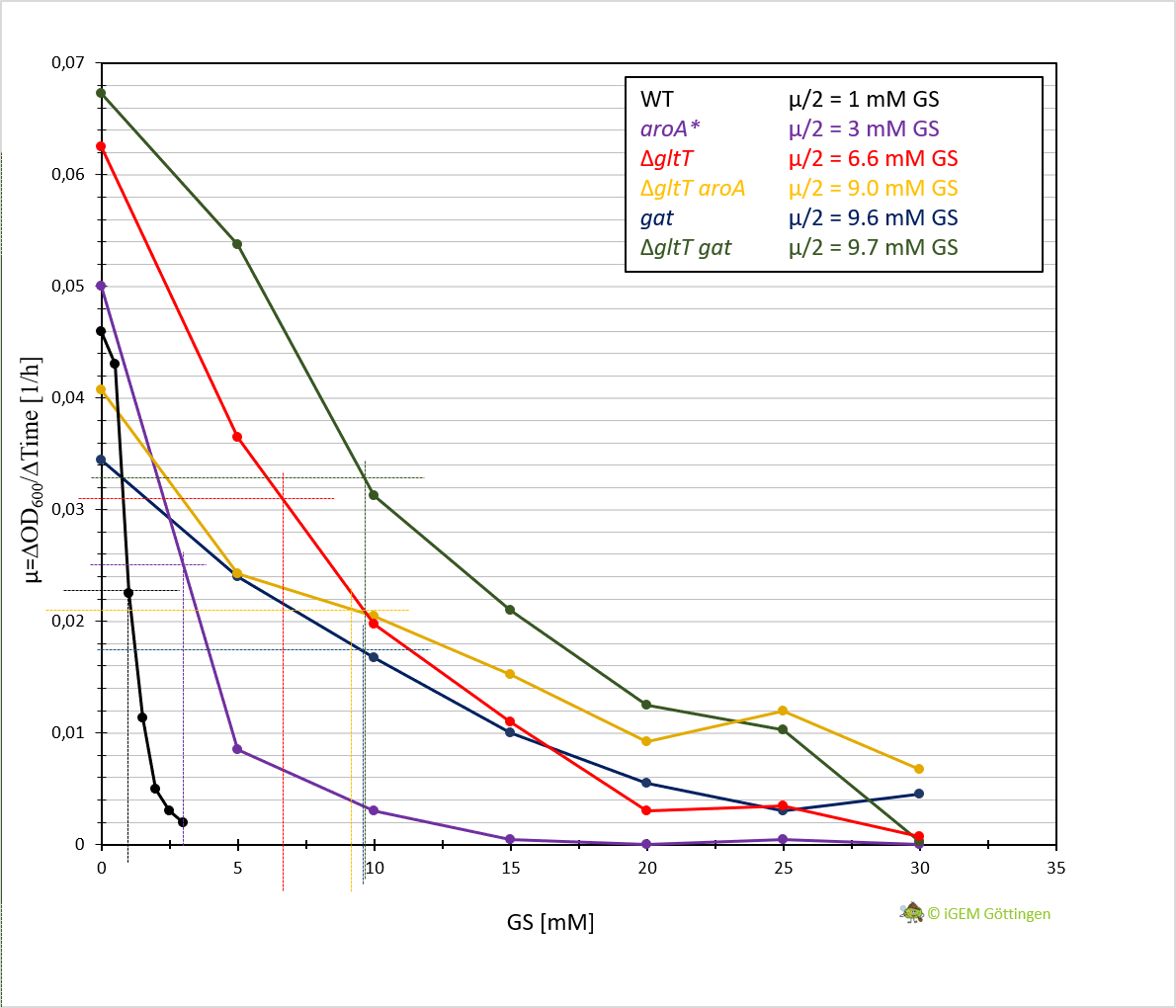
| + | [[File:T--Goettingen--ratearoagat.png|400px|left|thumb|'''Fig. 4.''' The relationship between the growth rate (µ) and the glyphosate (GS) concentration for the <i>B. subtilis</i> wild type (WT) strain SP1, the <i>ΔgltT</i> mutant strain BP233, the <i>gat</i> B. subtilis strain, the <i>aroA* B. subtilis</i> strain, the <i>B. subtilis</i> strain containing both <i>∆gltT</i> and <i>aroA*</i> and the the <i>B. subtilis</i> strain containing both <i>∆gltT</i> and <i>gat</i> in CS-Glc minimal medium supplemented with increasing amounts of glyphosate (GS). ]] | ||
| + | <html> | ||
| + | <hr> | ||
| + | </html> | ||
Revision as of 10:28, 9 October 2018
GAT: Glyphosate N-Acetyltransferase
This part encodes for an enzyme that n-acetylates the herbicide and antibiotic glyphosate.
The GAT-enzyme is able to confer glyphosate resistance to organisms such as Escherichia coli, Arabidopsis, tobacco and maize.
The acetylation of glyphosate may provide a new and alternative strategy in glyphosate support on crop plants.
We integrated this part into soil bacterium Bacillus subtilis to enable the bacteria to inactivate glyphosate, which would otherwise be deadly to them.
| Molecular weight | 17 kDa |
| Protein length | 146 aa |
| Gene length | 483 bp |
| Function | mediates glyphosate tolerance |
| Product | N-acetyl glyphosate |
| Ideal pH | 6.8 |
| Essential | no |
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Characterization
Engineering bacteria to disarm glyphosate
Many years ago it has been found that glyphosate can be inactivated by acetylation yielding N-acetylglyphosate ( Castle et al.,2004). Because the acetylated form of glyphosate has low affinity for the active site of EPSP synthase, it is nonherbicidal. The glyphosate N-acetyltransferase GAT from the Gram-positive soil bacterium Bacillus licheniformis transfers the acetyl group from acetyl-CoA onto the amino group of the weedkiller ( Castle et al.,2004).
Interestingly, B. subtilis possesses a putative glyphosate N-acetyltransferase, which is designated as YitI and shares 59% overall amino acid identity with GAT of B. licheniformis. However, the native GAT enzymes are very poor catalysts for N-acetylation of glyphosate ( Castle et al.,2004). Moreover, despite extensive screening of biological amines, including amino acids, nucleotides and antibiotics, the physiological substrates for the native enzymes are unknown (Siehl et al., 2007 JBC). As an alternative strategy for glyphosate resistance involving enzymatic conversion of glyphosate to N-acetylglyphosate the GAT was also subjected to directed evolution for creating an enzyme with higher efficiency and increased specificity for the herbicide ( Castle et al.,2004). When introduced into plants, optimized gat genes confer robust tolerance to glyphosate ( Castle et al.,2004). Therefore, we expect that bacteria like B. subtilis expressing the optimized GAT should tolerate high amounts of glyphosate even though it is taken up from the environment via the high-affinity glyphosate transporter GltT (see above). To evaluate the potential of the GAT to allow B. subtilis in the presence of glyphosate concentrations that are toxic for the wild type bacteria, we wanted to express the gat gene in B. subtilis (Figure 1).
GAT confers tolerance to glyphosate
We ordered the gat gene as a g-Block from Integrated DNA Technologies, which was free of charge because the company supports the iGEM competition. Next, we amplified the gat gene by PCR using the g-Block as template DNA. An artificial promoter and a ribosome binding site was attached during the PCR (Figure 1). The promoter-gene fusion was cloned and introduced in single copy into the genomes of the B. subtilis wild type strain and the gltT mutant strain to further increase its glyphosate tolerance (Figure 2). To assess the potential of the GAT to disarm glyphosate in the different B. subtilis strains, we propagated the bacteria on CS-Glc minimal medium agar plates that were supplemented with different amounts of glyphosate. As shown in Figure 2, the strains synthesizing the GAT tolerated significantly more glyphosate than the wild type strain. Moreover, the inactivation of the gltT glyphosate transporter gene and the overexpression of the gat gene confers high-level glyphosate tolerance to the bacteria. Thus, when introduced into B. subtilis , the optimized gat gene confers indeed robust tolerance to glyphosate. To conclude, the engineered bacteria possess two interesting properties: (i) B. subtilis promotes plant growth by protecting plants against pathogens and (ii), the bacteria take up glyphosate from the environment and inactivate the weedkiller!
Characeterization of GAT conferred tolerance to glyphosate
Cultivation of the different strains in liquid media allows more detailed characterisation. We analysed growth of the bacteria in CS-Glu medium supplemented with increasing amounts of glyphosate, using a platereader.
In general, the analysis supports previous results. The strain containing aroA* confers only a slight increase in resistance in comparison to the WT strain and hence a significantly lower resistance in comparison the ∆gltT strain (Figure 3 A, B and D ). However, for the strain combining aroA* and ∆gltT a 9-fold higher glyphosate concentration is needed to reduce the growth rate by 50% (Figure 3 F. Figure Y ).
Further characterisation of the Gat enzyme supports previous results and underline significant increased resistance in both strains conferring gat or a combination of gat and ∆gltT (Figure 3 C and E, Figure 4).