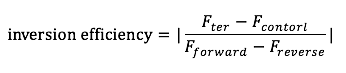Difference between revisions of "Part:BBa K2243031"
(→Characterization) |
|||
| Line 4: | Line 4: | ||
To test the influence of attB/P sites of phiC311 to terminator ECK120034435 (abbreviation: 435) in the forward direction. | To test the influence of attB/P sites of phiC311 to terminator ECK120034435 (abbreviation: 435) in the forward direction. | ||
| − | |||
| − | |||
| − | |||
<!-- --> | <!-- --> | ||
| − | Usage | + | <h2>Usage</h2> |
We constructed this part to characterize the recombination efficiency of the recombinase Streptomyces bacteria phage phiC31. It consists of the terminator ECK120034435 (abbreviation: 435) in the forward direction flanked by attB and attP sites of recombinase phiC31. Upon recombination, the orientation of the terminator changes. As a result, expression of downstream sequence is initiated. | We constructed this part to characterize the recombination efficiency of the recombinase Streptomyces bacteria phage phiC31. It consists of the terminator ECK120034435 (abbreviation: 435) in the forward direction flanked by attB and attP sites of recombinase phiC31. Upon recombination, the orientation of the terminator changes. As a result, expression of downstream sequence is initiated. | ||
<!-- --> | <!-- --> | ||
| − | Biology | + | <h2>Biology</h2> |
The attP site of phiC31 is used to integrate phage DNA at the host attB site of Streptomyces bacterium smegmatis, generating the recombinant junctions attL and attR. DNA cleavage and re-ligation occur at the central crossover region at attB and attP, which allows the sequence to be flipped, excised, or inserted between recognition sites. We obtained the terminator, attB and attP sites by oligo synthesis. | The attP site of phiC31 is used to integrate phage DNA at the host attB site of Streptomyces bacterium smegmatis, generating the recombinant junctions attL and attR. DNA cleavage and re-ligation occur at the central crossover region at attB and attP, which allows the sequence to be flipped, excised, or inserted between recognition sites. We obtained the terminator, attB and attP sites by oligo synthesis. | ||
<!-- --> | <!-- --> | ||
| − | + | <h2>Characterization</h2> | |
1. We first characterized the terminator strength using the following formula: | 1. We first characterized the terminator strength using the following formula: | ||
| Line 26: | Line 23: | ||
Ts=〖GFP〗of the random sequence/〖[GFP]〗with the terminator | Ts=〖GFP〗of the random sequence/〖[GFP]〗with the terminator | ||
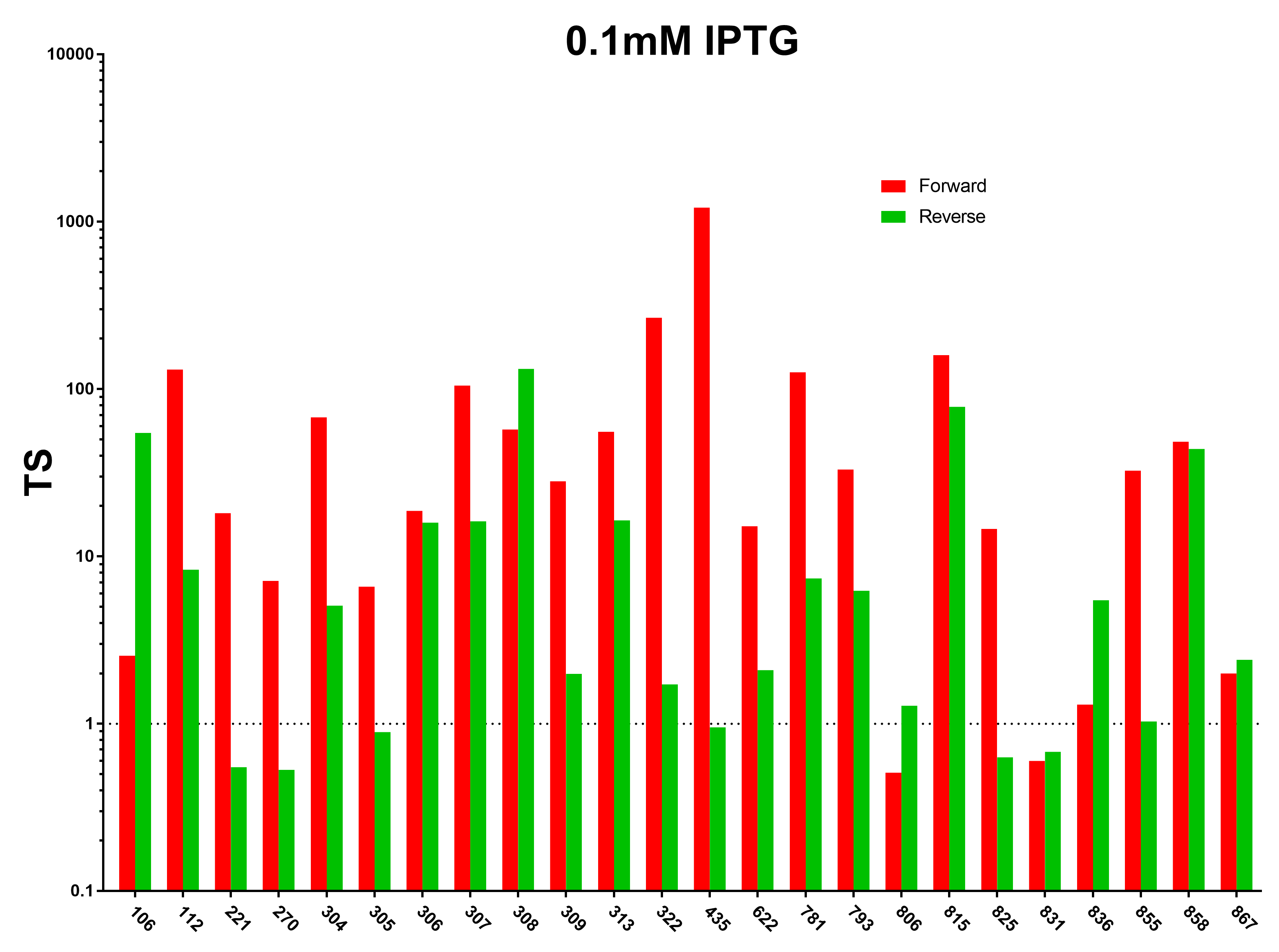
| − | [[File:Peking_TS_0.1mM.png|800px|thumb|center|Terminator Strength Induced with 0.1mM IPTG]] | + | [[File:Peking_TS_0.1mM.png|800px|thumb|center|Fig.1 Terminator Strength Induced with 0.1mM IPTG]] |
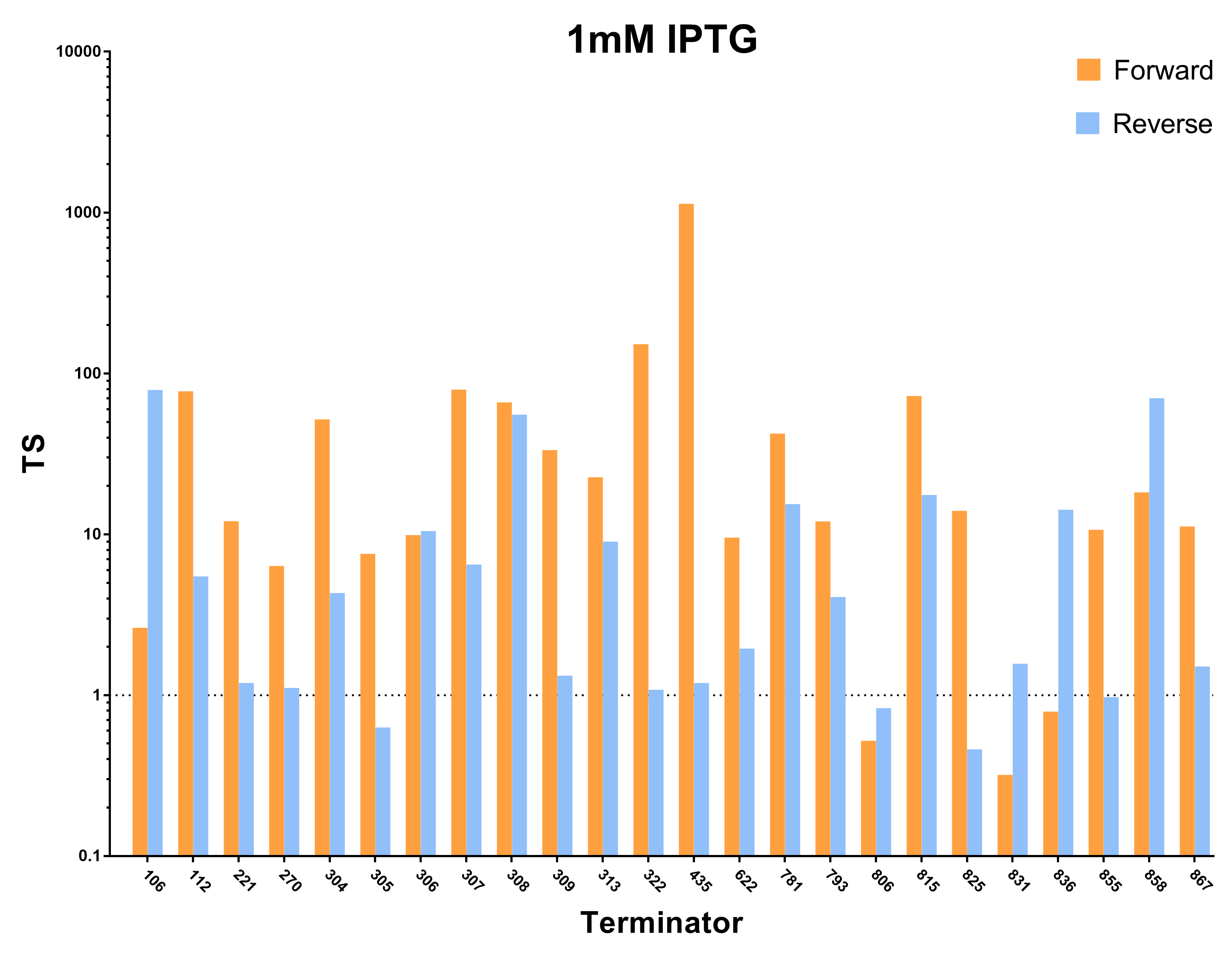
| − | [[File:Peking_TS_1mM.png|800px|thumb|center|Terminator Strength Induced with 1mM IPTG]] | + | [[File:Peking_TS_1mM.png|800px|thumb|center|Fig.2 Terminator Strength Induced with 1mM IPTG]] |
And we sifted out 6 desirable unidirectional terminators without potential cryptic promotor in both orientations. | And we sifted out 6 desirable unidirectional terminators without potential cryptic promotor in both orientations. | ||
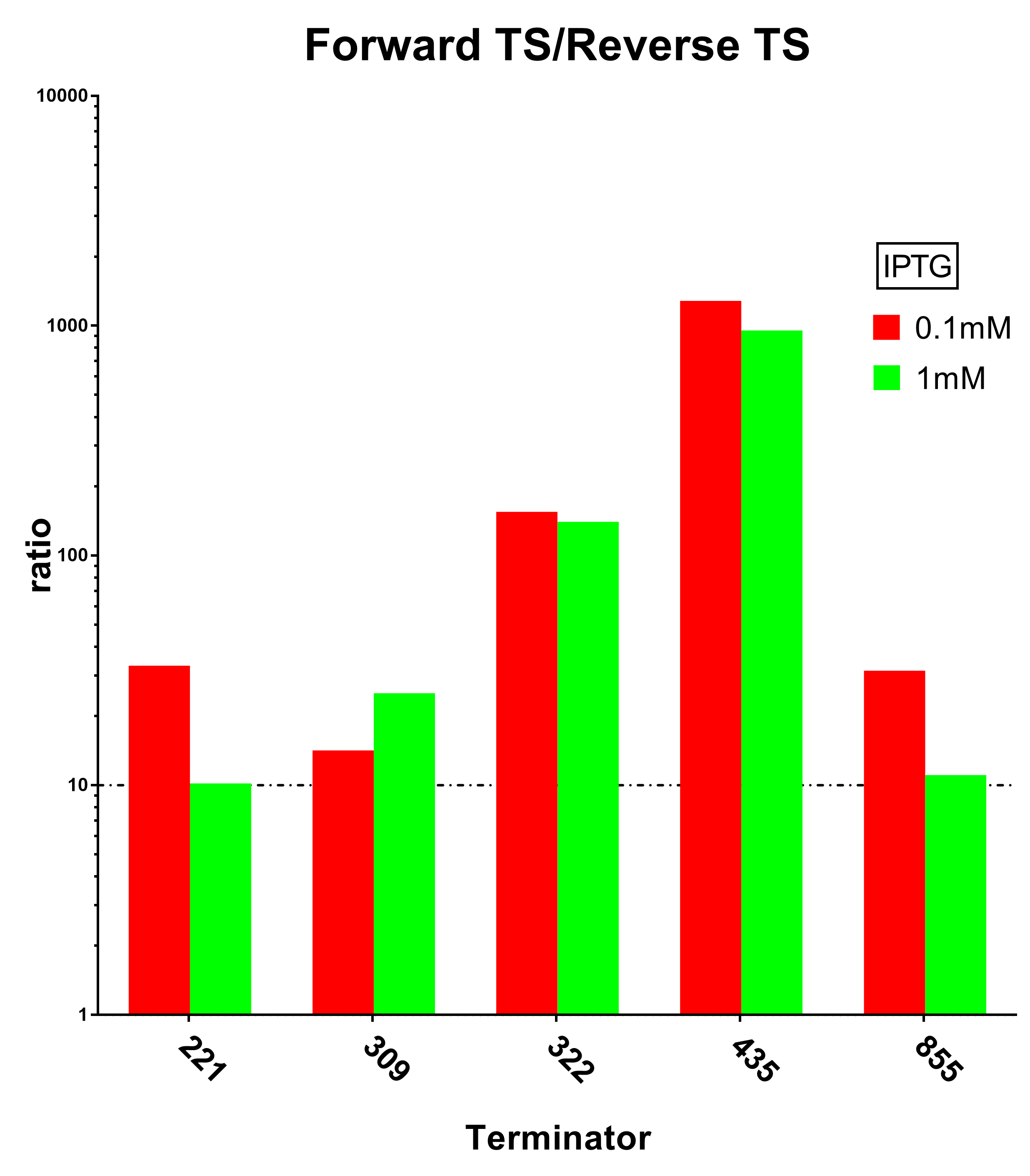
| − | [[File:Peking ratio.png|600px|thumb|center|Terminator Strength Induced with 0.1M IPTG]] | + | [[File:Peking ratio.png|600px|thumb|center|Fig.3 Terminator Strength Induced with 0.1M IPTG]] |
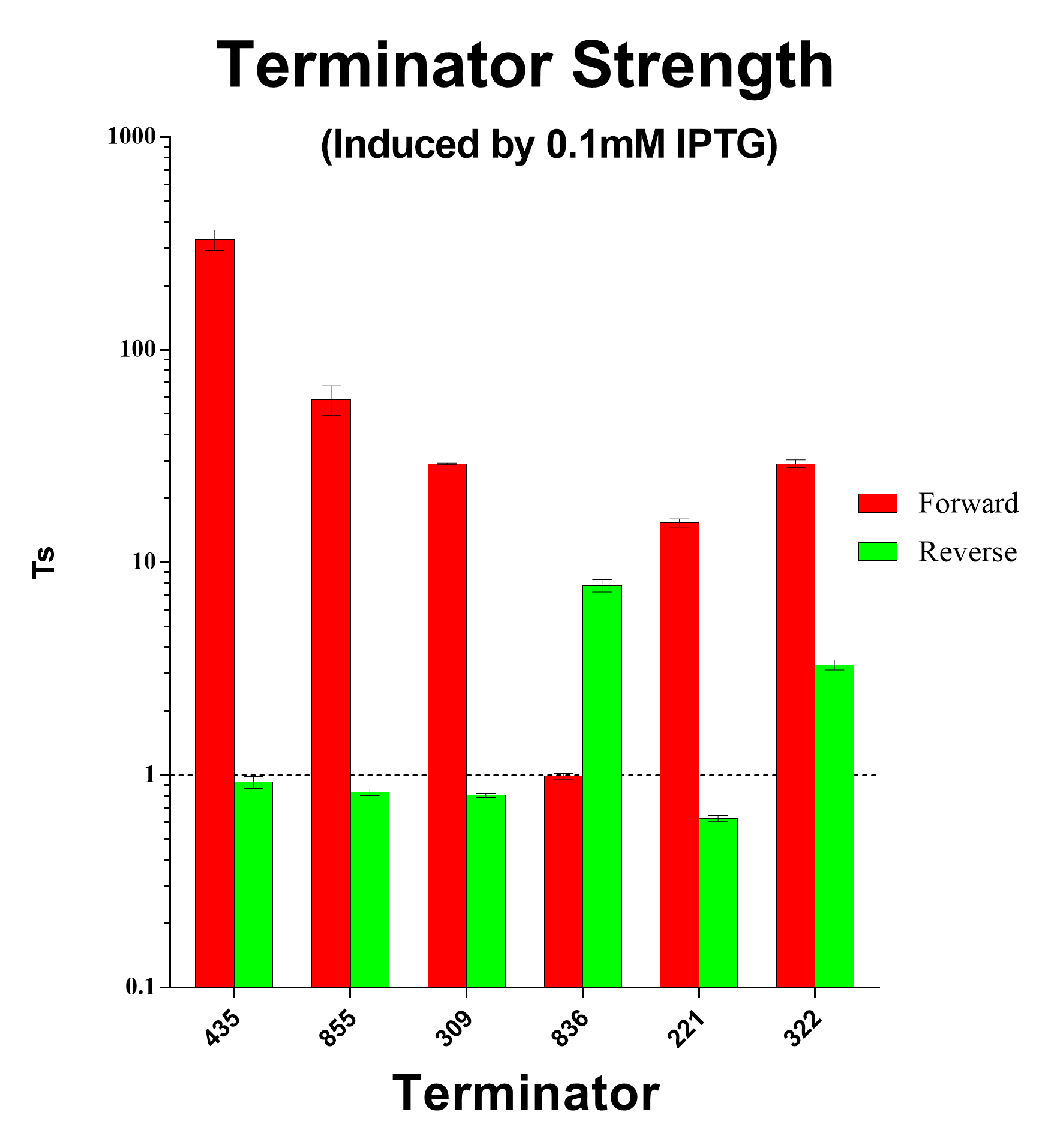
| − | [[File:Peking TS good terminators.png|600px|thumb|center|Forward and Reverse Terminator Strength Ratio Induced with 0.1mM and 1mM IPTG]] | + | [[File:Peking TS good terminators.png|600px|thumb|center|Fig.4 Forward and Reverse Terminator Strength Ratio Induced with 0.1mM and 1mM IPTG]] |
2. We then characterized the inversion efficiency of phiC31. | 2. We then characterized the inversion efficiency of phiC31. | ||
| Line 42: | Line 39: | ||
Microplate spectrophotometer was used to conduct preliminary measurements. Bacterial culture was added into 96-well plate(200ul for each well). OD600 and fluorescence intensity were measured. Background OD600 and fluorescence of plate, culture medium ,and autofluorescence should be eliminated through setting control groups. Fluorescence intensity/OD600 was cauculated using the net fluorescence intensity and net OD600. The transcription barrier strength of attBP/Terminator can be characterized quantitatively referring to the Ts formula. | Microplate spectrophotometer was used to conduct preliminary measurements. Bacterial culture was added into 96-well plate(200ul for each well). OD600 and fluorescence intensity were measured. Background OD600 and fluorescence of plate, culture medium ,and autofluorescence should be eliminated through setting control groups. Fluorescence intensity/OD600 was cauculated using the net fluorescence intensity and net OD600. The transcription barrier strength of attBP/Terminator can be characterized quantitatively referring to the Ts formula. | ||
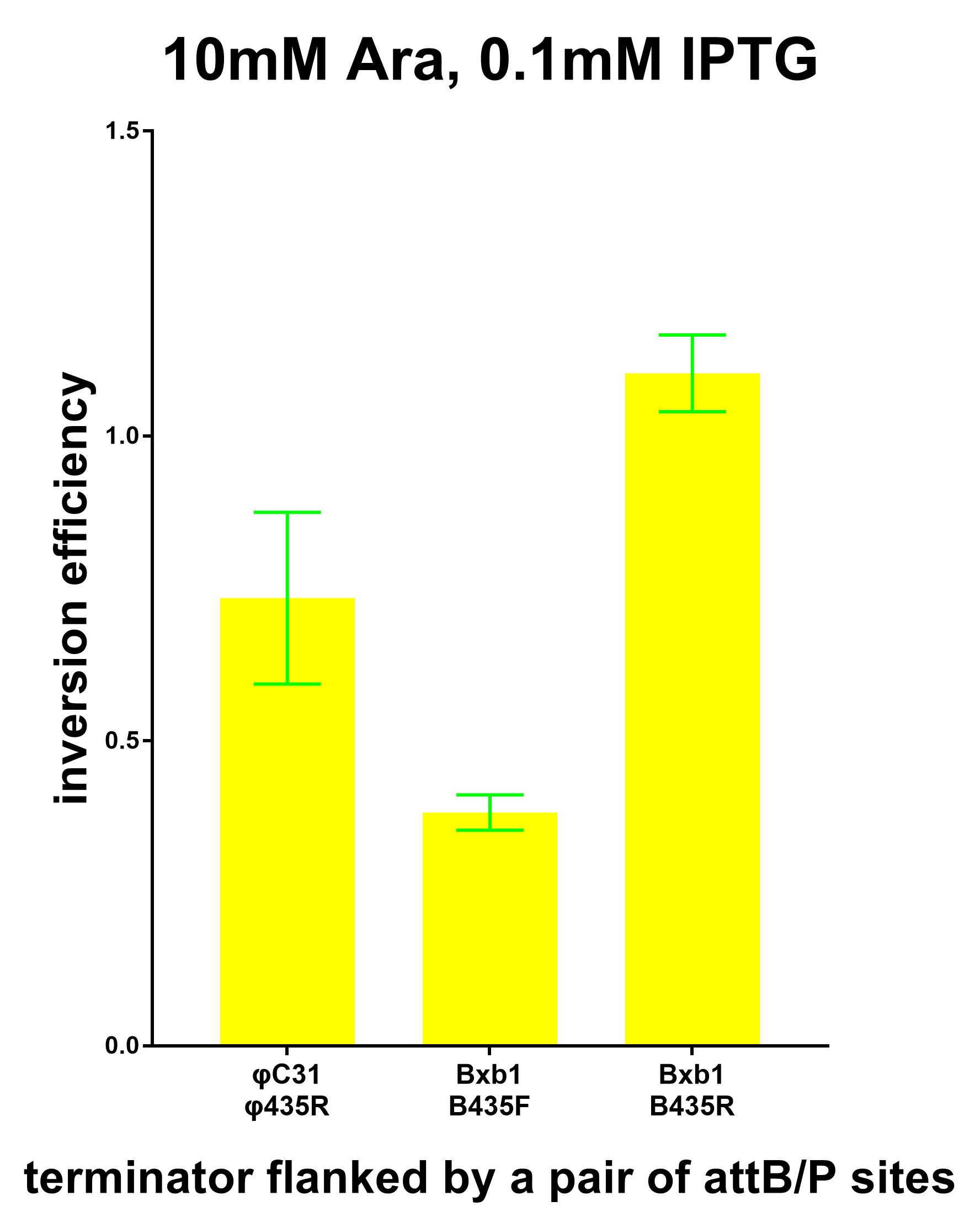
| − | [[File:Peking_1pair.png|600px|thumb|center|Terminators flanked by one pair of attB/P sites Induced with 0.1M IPTG and 10mM Arabinose]] | + | [[File:Peking_1pair.png|600px|thumb|center|Fig.5 Terminators flanked by one pair of attB/P sites Induced with 0.1M IPTG and 10mM Arabinose]] |
The inversion efficiency was calculated according to the formula below. | The inversion efficiency was calculated according to the formula below. | ||
[[File:Peking_ie.png|600px|center]] | [[File:Peking_ie.png|600px|center]] | ||
| − | |||
<!-- --> | <!-- --> | ||
| − | Terminator Reference Table | + | <h2>Terminator Reference Table</h2> |
[[Media:Peking_trt.xlsx]] | [[Media:Peking_trt.xlsx]] | ||
Revision as of 15:31, 1 November 2017
phiC31 attB_435F_phiC31 attP
To test the influence of attB/P sites of phiC311 to terminator ECK120034435 (abbreviation: 435) in the forward direction.
Usage
We constructed this part to characterize the recombination efficiency of the recombinase Streptomyces bacteria phage phiC31. It consists of the terminator ECK120034435 (abbreviation: 435) in the forward direction flanked by attB and attP sites of recombinase phiC31. Upon recombination, the orientation of the terminator changes. As a result, expression of downstream sequence is initiated.
Biology
The attP site of phiC31 is used to integrate phage DNA at the host attB site of Streptomyces bacterium smegmatis, generating the recombinant junctions attL and attR. DNA cleavage and re-ligation occur at the central crossover region at attB and attP, which allows the sequence to be flipped, excised, or inserted between recognition sites. We obtained the terminator, attB and attP sites by oligo synthesis.
Characterization
1. We first characterized the terminator strength using the following formula:
Ts=〖GFP〗of the random sequence/〖[GFP]〗with the terminator
And we sifted out 6 desirable unidirectional terminators without potential cryptic promotor in both orientations.
2. We then characterized the inversion efficiency of phiC31.
We transformed the testing system containing terminator ECK120034435 (abbreviation: 435) in the forward direction and expression system of phiC31 recombinase into one single cell to see if the inversions happened, and if the leakage expression of phiC31 would impact the system.
Microplate spectrophotometer was used to conduct preliminary measurements. Bacterial culture was added into 96-well plate(200ul for each well). OD600 and fluorescence intensity were measured. Background OD600 and fluorescence of plate, culture medium ,and autofluorescence should be eliminated through setting control groups. Fluorescence intensity/OD600 was cauculated using the net fluorescence intensity and net OD600. The transcription barrier strength of attBP/Terminator can be characterized quantitatively referring to the Ts formula.
The inversion efficiency was calculated according to the formula below.
Terminator Reference Table
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 4
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI site found at 24
Illegal BsaI.rc site found at 155