Difference between revisions of "Part:BBa K2201017"
| (One intermediate revision by the same user not shown) | |||
| Line 2: | Line 2: | ||
__NOTOC__ | __NOTOC__ | ||
<partinfo>BBa_K2201017 short</partinfo> | <partinfo>BBa_K2201017 short</partinfo> | ||
| + | ===Usage and Biology=== | ||
| + | This plasmid was designed as a backbone for the incorporation of fragments containing unnatural base pairs. | ||
| + | Given that selection and screening of the transformants was not possible with the experimental design, a plasmid which would kill or inhibit growth of the wrong colonies was designed. The initial design focused on the use of CcdB, but given that <i>ccd</i>B cannot be handed in, levansucrase of <i>Bacillus subtilis</i>, encoded by <i>sac</i>B, was used instead. Therefore, a <i>mRFP-sac</i>B fusion construct was constructed. BBa_J23100 was used as a promotor and BBa_B0034 as a strong RBS, followed by BBa_E1010. Between <i>mRFP</i> and <i>sac</i>B a linker consisting of four alanines was used. Given that this plasmid was designed to eventually carry an unnatural base pair, a low-copy plasmid was the better choice for a higher stability of the unnatural base pair. Therefore, the backbone pSB3C5 was used. | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
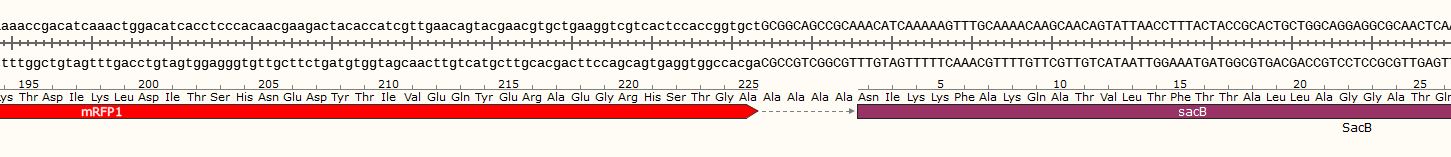
<span class='h3bb'>Sequence and Features</span> | <span class='h3bb'>Sequence and Features</span> | ||
<partinfo>BBa_K2201017 SequenceAndFeatures</partinfo> | <partinfo>BBa_K2201017 SequenceAndFeatures</partinfo> | ||
| + | __TOC__ | ||
| + | ==Plasmid Design== | ||
| + | [[File:MrfpsacBconstruct.jpeg|800px|thumb|center|<b>Figure (1): Fusionprotein of mRFP and SacB.</b>A flexible 4xAla linker was used between mRFP and SacB.]] | ||
| + | [[File:mrfpsacBcloning.jpeg|380px|thumb|left|<b>Figure (2):Method for selection of the correct plasmids in liquid cultures.</b>If the assembly was successful, cells turn red and survive in sucrose supplemented media. The cells die if the assembly was not successful, since <i>sac</i>B can be expressed.]] | ||
| + | <html> | ||
| + | <p align="justify"> | ||
| + | In the initial plasmid, <i>mRFP</i> and <i>sac</i>B are in-frame, meaning that cells turn red when incubated in standard media such as LB, but die or grow weakly when cultivated in media supplemented with sucrose. This is due to the toxic effect of levansucrase which unfolds when cells are cultivated in sucrose supplemented media. | ||
| + | The linker between <i>mRFP</i> and <i>sac</i>B represents the insertion site for the fragment containing the unnatural base pair. This insertion, if successful, leads to a frameshift of the downstream located sacB, leading to loss of function. Therefore, cells can only survive in sucrose supplemented media if the fragment containing the unnatural base pair was successfully incorporated. This provides an easy screening method, since cells that turn red and survive in sucrose supplemented media most likely inserted the fragment containing the unnatural base pair into the plasmid. Having the coding sequences of both proteins in one frame has one big advantage: given that sacB is prone to loss-of-function mutations, contaminations can be easily distinguished by color. Furthermore, cells with mutations in the promotor region would also survive since they would not express sacB, but not turn red. Therefore, this two-layered system allows to visually check if a culture is contaminated, has a mutation in the promotor region or if the correct plasmid is present. | ||
| + | </p> | ||
| + | <br style="clear: both" /> | ||
| + | </html>[[File:MrfpsacBcultivation.jpeg|500px|thumb|right|<b>Figure (3):Cultivation of E. coli BL21(DE3) in LB media supplemented with different concentrations of sucrose.</b> A clear difference in growth can be observed, with higher sucrose concentrations leading to weaker growth. The mRFP-SacB fusion protein does not lead to cell death, but shows bacteriostatic properties. Therefore, cells that successfully integrated the fragment containing the unnatural base pair should grow much better in the selective media and therefore overgrow cells harboring religated plasmid backbones.]] | ||
| + | <html> | ||
| + | <p align="justify"> | ||
| + | To test the effect of sucrose on growth of the cells, a cultivation was performed of <i>E. coli</i> BL21(DE3) harboring BBa_K2201017. The cultivations were carried out in a 12 well plate in 1 mL of LB media supplemented with different concentrations of sucrose. Three biological replicates were cultivated for each condition and three technical replicates taken for each measurement point. | ||
| + | </p> | ||
| + | <br style="clear: both" /> | ||
| + | </html> | ||
| + | [[File:CulivationmrfpsacBplateliquid.jpeg|300px|thumb|left|<b> Figure (4): <i>E. coli</i> BL21(DE3) BBa_K2201017 cultivated on plates and in liquid cultures with and without 10% sucrose.</b> All cultures were cultivated for 24 hours at 37 °C. A) Shows a drop on a LB agar plate supplemented with 10 % sucrose. B) Shows a drop on a LB agar plate without sucrose. A clear difference can be observed between the two drops. In presence of sucrose, <i>E. coli</i> BL21(DE3) BBa_K2201017 grew much weaker and did not turn red. Without sucrose, the cells grew much better and turned red. C) The same difference in growth and color could be observed in liquid cultures.]] | ||
| + | <html> | ||
| + | <p align="justify"> | ||
| + | The cultivations shown in figure (3) clearly show that sucrose has a significant effect on growth of strains carrying BBa_K2201017. A higher sucrose concentration leads to weaker growth, with strains cultivated in 20 % sucrose reaching only ~33 % of the OD600 of the reference strain cultivated in sucrose free media. The reference reached a final optical density of 3.233 ± 0.148, strains cultivated in media supplemented with 10 % sucrose 1.527 ± 0.055 and strains cultivated in 20 % sucrose reached a final optical density of 0.44 ± 0.05. Figure (4) shows a comparison of strains cultivated in media without sucrose and media supplemented with 10 % sucrose. A preculture of E. coli BL21(DE3) harboring BBa_K2201017 was prepared and cultivated overnight at 37 °C. 30 µl of this precultures were used and dropped onto LB agar plates either without or supplemented with 10 % sucrose. 50 µl of the same preculture were used to inoculate 3 mL of LB media without sucrose and 3 mL of LB media supplemented with 10 % sucrose. | ||
| + | </p> | ||
| + | <br style="clear: both" /> | ||
| + | </html> | ||
<!-- Uncomment this to enable Functional Parameter display | <!-- Uncomment this to enable Functional Parameter display | ||
Latest revision as of 12:50, 1 November 2017
Construct for the integration of unnatural base pairs
Usage and Biology
This plasmid was designed as a backbone for the incorporation of fragments containing unnatural base pairs. Given that selection and screening of the transformants was not possible with the experimental design, a plasmid which would kill or inhibit growth of the wrong colonies was designed. The initial design focused on the use of CcdB, but given that ccdB cannot be handed in, levansucrase of Bacillus subtilis, encoded by sacB, was used instead. Therefore, a mRFP-sacB fusion construct was constructed. BBa_J23100 was used as a promotor and BBa_B0034 as a strong RBS, followed by BBa_E1010. Between mRFP and sacB a linker consisting of four alanines was used. Given that this plasmid was designed to eventually carry an unnatural base pair, a low-copy plasmid was the better choice for a higher stability of the unnatural base pair. Therefore, the backbone pSB3C5 was used.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 7
Illegal NheI site found at 30 - 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal AgeI site found at 616
Illegal AgeI site found at 728 - 1000COMPATIBLE WITH RFC[1000]
Contents
Plasmid Design
In the initial plasmid, mRFP and sacB are in-frame, meaning that cells turn red when incubated in standard media such as LB, but die or grow weakly when cultivated in media supplemented with sucrose. This is due to the toxic effect of levansucrase which unfolds when cells are cultivated in sucrose supplemented media. The linker between mRFP and sacB represents the insertion site for the fragment containing the unnatural base pair. This insertion, if successful, leads to a frameshift of the downstream located sacB, leading to loss of function. Therefore, cells can only survive in sucrose supplemented media if the fragment containing the unnatural base pair was successfully incorporated. This provides an easy screening method, since cells that turn red and survive in sucrose supplemented media most likely inserted the fragment containing the unnatural base pair into the plasmid. Having the coding sequences of both proteins in one frame has one big advantage: given that sacB is prone to loss-of-function mutations, contaminations can be easily distinguished by color. Furthermore, cells with mutations in the promotor region would also survive since they would not express sacB, but not turn red. Therefore, this two-layered system allows to visually check if a culture is contaminated, has a mutation in the promotor region or if the correct plasmid is present.

To test the effect of sucrose on growth of the cells, a cultivation was performed of E. coli BL21(DE3) harboring BBa_K2201017. The cultivations were carried out in a 12 well plate in 1 mL of LB media supplemented with different concentrations of sucrose. Three biological replicates were cultivated for each condition and three technical replicates taken for each measurement point.

The cultivations shown in figure (3) clearly show that sucrose has a significant effect on growth of strains carrying BBa_K2201017. A higher sucrose concentration leads to weaker growth, with strains cultivated in 20 % sucrose reaching only ~33 % of the OD600 of the reference strain cultivated in sucrose free media. The reference reached a final optical density of 3.233 ± 0.148, strains cultivated in media supplemented with 10 % sucrose 1.527 ± 0.055 and strains cultivated in 20 % sucrose reached a final optical density of 0.44 ± 0.05. Figure (4) shows a comparison of strains cultivated in media without sucrose and media supplemented with 10 % sucrose. A preculture of E. coli BL21(DE3) harboring BBa_K2201017 was prepared and cultivated overnight at 37 °C. 30 µl of this precultures were used and dropped onto LB agar plates either without or supplemented with 10 % sucrose. 50 µl of the same preculture were used to inoculate 3 mL of LB media without sucrose and 3 mL of LB media supplemented with 10 % sucrose.