Difference between revisions of "Part:BBa K1949101:Design"
| Line 21: | Line 21: | ||
-Plasmids | -Plasmids | ||
| − | + | <i>E. coli</i> A: Pcon - <i>rbs</i> - <i>gfp</i> (pSB6A1), Plac - <i>rbs</i> (pSB3K3) | |
| − | + | <i>E. coli</i> B: PBAD - <i>rbs - mazF - tt</i> - Pcon - <i>rbs - gfp</i> (pSB6A1), Plac - <i>rbs</i> (pSB3K3) | |
=====Ⅱ.<i>mazEF</i> System Assay ~Stop & GO~===== | =====Ⅱ.<i>mazEF</i> System Assay ~Stop & GO~===== | ||
| Line 29: | Line 29: | ||
-Plasmids | -Plasmids | ||
| − | + | <i>E. coli</i> C: PBAD - <i>rbs</i> (pSB6A1), Plac - <i>rbs</i> (pSB3K3) | |
| − | + | <i>E. coli</i> A: Pcon - <i>rbs - gfp</i> (pSB6A1), Plac - <i>rbs</i> (pSB3K3) | |
| − | + | <i>E. coli</i> D: PBAD - <i>rbs - mazF - tt</i> - Pcon - <i>rbs - gfp</i> (pSB6A1), Plac - <i>rbs - mazE</i> (pSB3K3) | |
| + | |||
| + | <i>E. coli</i> B: PBAD - <i>rbs - mazF - tt</i> - Pcon - <i>rbs - gfp</i> (pSB6A1), Plac - <i>rbs</i> (pSB3K3) | ||
| − | |||
=====Ⅲ.<i>mazEF</i> System Assay ~Go & Stop~===== | =====Ⅲ.<i>mazEF</i> System Assay ~Go & Stop~===== | ||
| Line 41: | Line 42: | ||
-Plasmids | -Plasmids | ||
| − | + | <i>E. coli</i> C: PBAD - <i>rbs</i> (pSB6A1), Plac - <i>rbs</i> (pSB3K3) | |
| − | + | <i>E. coli</i> A: Pcon - <i>rbs - gfp</i> (pSB6A1), Plac - <i>rbs</i> (pSB3K3) | |
| − | + | <i>E. coli</i> G: PBAD - <i>rbs - mazF - tt</i> - Pcon - <i>rbs - gfp</i> (pSB6A1), Pcon - <i>rbs</i>(weak) - <i>mazE</i> (pSB3K3) | |
| − | + | <i>E. coli</i> F: PBAD - <i>rbs - mazF - tt</i> - Pcon - <i>rbs - gfp</i> (pSB6A1), Pcon - <i>rbs - mazE</i> (pSB3K3) | |
| − | + | <i>E. coli</i> E: PBAD - <i>rbs - mazF - tt</i> - Pcon - <i>rbs - gfp</i> (pSB6A1), vector (pSB3K3) | |
=====Ⅳ.Control of Cell Growth===== | =====Ⅳ.Control of Cell Growth===== | ||
| Line 55: | Line 56: | ||
-Plasmid | -Plasmid | ||
| − | + | <i>E. coli</i> i: PBAD - <i>rbs</i> (pSB6A1), Plac - <i>rbs</i> (pSB3K3) | |
| − | + | <i>E. coli</i> D: PBAD - <i>rbs - mazF</i> (pSB6A1), Plac - <i>rbs - mazE</i> (pSB3K3) | |
| − | + | <i>E. coli</i> H: PBAD - <i>rbs - mazF</i>(pSB6A1), Plac - <i>rbs</i> (pSB3K3) | |
====Assay protocol==== | ====Assay protocol==== | ||
| Line 117: | Line 118: | ||
5)Incubate with vigorous shaking for 24 h, and measure the turbidity and the RFU of GFP at proper times. | 5)Incubate with vigorous shaking for 24 h, and measure the turbidity and the RFU of GFP at proper times. | ||
| − | =====Ⅳ. | + | =====Ⅳ.Ⅳ.<i>mazEF</i> System Assay on the LB Agar Plate(Queen's Caprice)===== |
1)Making LB agar medium(see Table 1.).<br> | 1)Making LB agar medium(see Table 1.).<br> | ||
[[Image:Agar medium.jpg|center|600px]]<br> | [[Image:Agar medium.jpg|center|600px]]<br> | ||
Revision as of 20:08, 19 October 2016
Contents
PBAD-rbs-mazF
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 1205
- 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 1144
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal AgeI site found at 979
- 1000INCOMPATIBLE WITH RFC[1000]Illegal SapI site found at 961
Design Notes
sequence confirmed
Materials and Methods
Construction
-Strain
All the plasmids were prepared in XL1-Blue strain.
Ⅰ.Adjustment of MazF Expression
-Plasmids
E. coli A: Pcon - rbs - gfp (pSB6A1), Plac - rbs (pSB3K3)
E. coli B: PBAD - rbs - mazF - tt - Pcon - rbs - gfp (pSB6A1), Plac - rbs (pSB3K3)
Ⅱ.mazEF System Assay ~Stop & GO~
-Plasmids
E. coli C: PBAD - rbs (pSB6A1), Plac - rbs (pSB3K3)
E. coli A: Pcon - rbs - gfp (pSB6A1), Plac - rbs (pSB3K3)
E. coli D: PBAD - rbs - mazF - tt - Pcon - rbs - gfp (pSB6A1), Plac - rbs - mazE (pSB3K3)
E. coli B: PBAD - rbs - mazF - tt - Pcon - rbs - gfp (pSB6A1), Plac - rbs (pSB3K3)
Ⅲ.mazEF System Assay ~Go & Stop~
-Plasmids
E. coli C: PBAD - rbs (pSB6A1), Plac - rbs (pSB3K3)
E. coli A: Pcon - rbs - gfp (pSB6A1), Plac - rbs (pSB3K3)
E. coli G: PBAD - rbs - mazF - tt - Pcon - rbs - gfp (pSB6A1), Pcon - rbs(weak) - mazE (pSB3K3)
E. coli F: PBAD - rbs - mazF - tt - Pcon - rbs - gfp (pSB6A1), Pcon - rbs - mazE (pSB3K3)
E. coli E: PBAD - rbs - mazF - tt - Pcon - rbs - gfp (pSB6A1), vector (pSB3K3)
Ⅳ.Control of Cell Growth
-Plasmid
E. coli i: PBAD - rbs (pSB6A1), Plac - rbs (pSB3K3)
E. coli D: PBAD - rbs - mazF (pSB6A1), Plac - rbs - mazE (pSB3K3)
E. coli H: PBAD - rbs - mazF(pSB6A1), Plac - rbs (pSB3K3)
Assay protocol
Ⅰ.Adjustment of MazF Expression
Pre-culture
1)Suspend colonies on a master plate into LB medium containing ampicillin (50 microg / mL) and kanamycin (50 microg / mL).
2)Incubate with vigorous shaking for 12 h.
Incubation and Assay
1)Measure the turbidity of the pre-cultures.
2)Dilute the pre- cultures to 1 / 30 into LB medium containing 4 mL ampicillin and kanamycin.
3)Incubate with vigorous shaking so that the turbidity becomes 0.03.
4)Add arabinose so that the final concentration becomes 0.2%, 0.02%, 0.002% 0.0002% and 0%.
5)Incubate with vigorous shaking for 24 h, and measure the turbidity and the RFU of GFP.
Ⅱ.mazEF System Assay ~Stop & GO~
Pre-culture
1)Suspend colonies on a master plate into LB medium containing ampicillin (50 microg / mL) and kanamycin (50 microg / mL).
2)Incubate with vigorous shaking for 12 h.
Incubation and Assay
1)Measure the turbidity of the pre-cultures.
2)Dilute the pre- cultures to 1 / 30 into LB medium containing 4 mL ampicillin and kanamycin.
3)Incubate with vigorous shaking so that turbidity becomes 0.03.
4)Add arabinose so that the final concentration becomes 0.02%.
5)Add IPTG until the concentration becomes 2 mM after adding arabinose.
6)Incubate with vigorous shaking for 24 h, and measure turbidity and RFU of GFP at the proper time.
Ⅲ.mazEF System Assay ~Go & Stop~
Pre-culture
1)Suspend colonies on a master plate into LB medium containing ampicillin (50 microg / mL) and kanamycin (50 microg / mL).
2)Incubate with vigorous shaking for 12 h.
Incubation and Assay
1)Measure the turbidity of the pre-cultures.
2)Dilute the pre- cultures to 1 / 30 into LB medium containing 4 mL ampicillin and kanamycin.
3)Incubate with vigorous shaking so that the turbidity becomes 0.03.
4)Add arabinose so that the final concentration becomes 0.02%.
5)Incubate with vigorous shaking for 24 h, and measure the turbidity and the RFU of GFP at proper times.
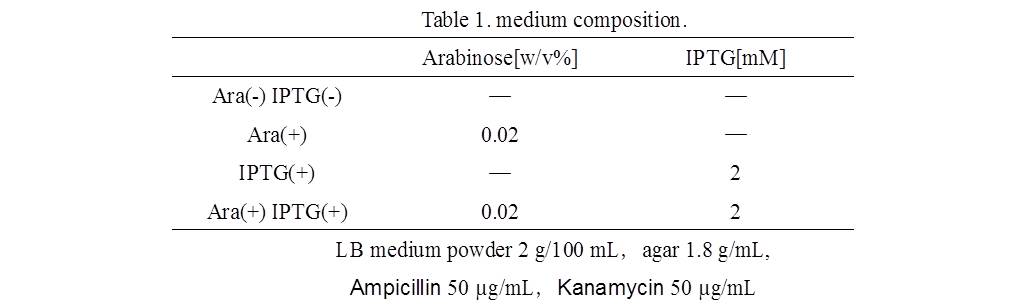
Ⅳ.Ⅳ.mazEF System Assay on the LB Agar Plate(Queen's Caprice)
1)Making LB agar medium(see Table 1.).
2)E. coli are applied at 3 agar medium (in arabinose, in IPTG, in arabinose and IPTG)(Fig. 1.).
3)Overnight culture at 37°C for 24 h.
4)To confirm TA system, inoculate colonies of E. coli having plasmids at agar medium containing arabinose and IPTG.
5)Overnight culture at 37°C for 24 h.
6)Inoculate colonies of E. coli into agar medium containing arabinose.
7)Overnight culture at 37°C for 24 h.
8)Inoculate colonies of E. coli into agar medium in arabinose and IPTG.
9)Overnight culture at 37°C for 24 h.
References
1)Hazan, R., B. Sat, and H. Engelberg-Kulka. Escherichia coli mazEF mediated cell death is triggered by various stressful conditions. J. Bacteriol.186:3663–3669.