Difference between revisions of "Part:BBa K1789004"
(→Enzyme cutting) |
(→Experimental Validation) |
||
| Line 25: | Line 25: | ||
==Experimental Validation== | ==Experimental Validation== | ||
| − | This part is validated through | + | This part is validated through three ways: enzyme cutting, PCR, Sequence, and functional testing |
===PCR=== | ===PCR=== | ||
Revision as of 13:06, 18 September 2015
GFP2
In order to check the functions of our system that whether two frames standing near enough so that enzymes could react easily, we divided the sequence of GFP into two parts called N-fragment and C-fragment. This is the latter. This works like this way: only when supplied with the proper distance for tow enzymes will it produce light output.
Usage and Biology
Bimolecular fluorescence complementation (BiFC) means two non-fluorescent complementary fragments of the fluorescent protein can reassemble to form a fluorescent complex and restore fluorescence when they are fused to two proteins that interact with each other.
BiFC analysis has been used to study interactions among a wide range of proteins in many cell types. The study of interactions and post-translational modification of the protein makes people master the biological regulatory mechanism more. Interactions in protein are also highly valued, so there have been a number of related technologies having different characteristics and applications[1-2].
Such as GFP,after the sequence of certain sites in interchanges with the amino-terminal or carboxy-terminal sequence loop, it could still be able to fold correctly to form the structure of the chromophore and maintain fluorescence properties[3-5].
When fusing the GFP2 with our TALE2/3, we found the additional T base on the 5’ terminal of the BBa_I715020 annoying because it may cause frameshift mutation if we just use the standard Bio-brick assembly to fuse two enzymes. And that mutation may disable the whole reporter gene. In order to meet this challenge and make this part easier to use, we designed a pair of prime to remove that additional base and make this part easier to be used.
Sequence and Features
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI.rc site found at 173
Experimental Validation
This part is validated through three ways: enzyme cutting, PCR, Sequence, and functional testing
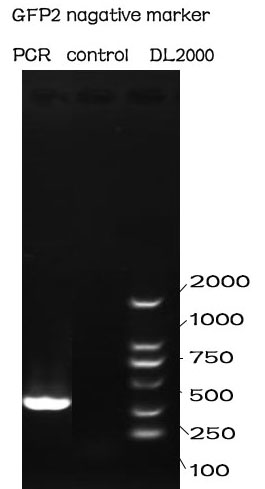
PCR
Methods
The PCR is performed with Premix EX Taq by Takara.
F-Prime: 5’-CGGAATTCGCGGCCGCTTCTAGAAAGAATGGAATCC-3’
R-Prime: 5’-GGACTAGTTTATTATTTGTATAGTTC -3’
The PCR protocol is selected based on the Users Manuel. The Electrophoresis was performed on a 1% Agarose glu.
Results
The result of the agarose electrophoresis was shown on the picture above.
Functional Test
This part is tested together with the part BBa_K1789003, in the device BBa_K178901.
The result shows that this part can shine with GFP1.
References
[ 1] Drewes G, Bouwmeester T.Global approaches to protein – protein interaction[ J ]. Curr Opin Cell Biol, 2003, 15(2): 199-205.
[ 2] Collura V, Boissy G. From protein -protein complexes to interactomics [ J ].Subcell Biochem, 2007, 43: 135-83.
[ 3] M isteli T, Spector DL. Application of the green fluorescent protein in cell biology and biotechnology[ J ].Nat Biotechnol, 1997, 15( 10): 961 - 964.
[ 4] Baird G. S, Zacharias DA, Tsien RY. Circular permutation and receptor insertion within green fluorescent proteins[ J ]. Proc Natl Acad Sci USA , 1999, 96( 20): 11241-11246.
[ 5] Ghosh I, Hamilton AD, Regan L. Antiparallel leucinezipper –directed protein reassembly: application to the green fluorescent protein[ J ]. J Am Chem Soc, 2000, 122: 5658-5659.