Difference between revisions of "Part:BBa K1688004"
| Line 26: | Line 26: | ||
[[File:Uppsala2015_dTomato_Chromatogram.png]] | [[File:Uppsala2015_dTomato_Chromatogram.png]] | ||
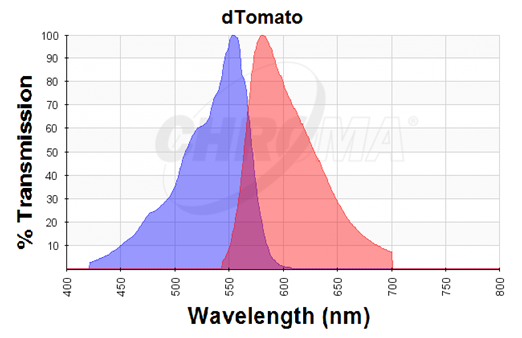
Figure 4. Excitation (blue curve) and emission (red curve) spectra for the dTomato fluorescent protein (the graph is designed with the following tool: https://www.chroma.com/spectra-viewer) | Figure 4. Excitation (blue curve) and emission (red curve) spectra for the dTomato fluorescent protein (the graph is designed with the following tool: https://www.chroma.com/spectra-viewer) | ||
| + | |||
| + | == Characterization == | ||
| + | |||
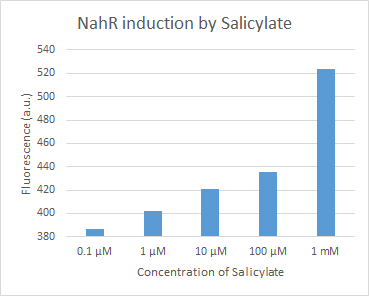
| + | We characterized dTomato by using the NahR/Psal promoter system on a pSB1C3 plasmid (See figure 1,2 and 3). The cells containing the plasmid were streaked on agar plates with different salicylate concentrations in the agar medium. Similarly, we also examined liquid cultures with different concentrations of salicylate in the solution. Both of these tests showed that the bacteria were producing the dTomato protein in presence of salicylate, independent of the salicylate being in liquid culture or in the solid growth medium. It can also be easily observed that the fluorescence increased with increasing salicylate concentrations (See figure 1 and 2). The measurements were made with the fluorometer that our team built in order to be able to characterize this system. These results were compared to MACS measurements on the same colonies and the measurements were correlated. | ||
| + | |||
== References == | == References == | ||
Nathan C Shaner, Robert E Campbell, Paul A Steinbach, Ben N G Giepmans, Amy E Palmer & Roger Y Tsien. “Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein”, 2004 | Nathan C Shaner, Robert E Campbell, Paul A Steinbach, Ben N G Giepmans, Amy E Palmer & Roger Y Tsien. “Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein”, 2004 | ||
Latest revision as of 02:09, 19 September 2015
dTomato (inc RBS)
Composite part consisting of the RBS, BBa_B0034, and the red fluorescent protein, dTomato (BBa_K1688019). dTomato has not been bricked previously.
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
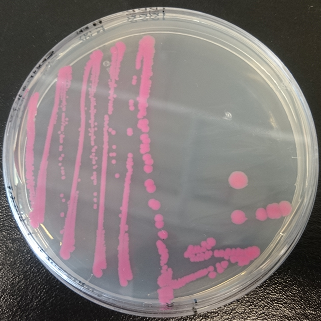
Figure 1. dTomato expressed in E. coli DH5-α with an inducible promoter.
Figure 2. dTomato expressed in E. coli DH5-alpha. NahR/Psal induction test in LB medium. Concentration of salicylate from left to right: 0,1 μM, 1 μM, 10 μM, 100 μM and 1 mM.
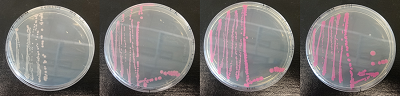
Figure 3. dTomato expressed in E. coli DH5-alpha. NahR/Psal induction test on agar plate. Concentration of salicylate from left to right: 0,1 μM, 1 μM, 10 μM, 100 μM and 1 mM.
Usage and Biology
The dTomato protein is a fluorescent protein dimer, created by direct evolution of the wild-type DsRed, from Discosoma sp. (Shaner et al, - Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein, 2004). The dTomato protein is a fluorescent dimer protein that emits orange-red light when it is excited by green-yellow light. It is preferable to use – especially in self-made fluorometry tests – because the excitation wavelengths and the emission wavelengths don't overlap as much as in other fluorescent proteins. The dTomato excitation peak is at 554 nm and 50% of it is at 510 nm. Also, its emission peak is at 581 nm and its 50% emission at 629 nm (Figure 4).
Figure 4. Excitation (blue curve) and emission (red curve) spectra for the dTomato fluorescent protein (the graph is designed with the following tool: https://www.chroma.com/spectra-viewer)
Characterization
We characterized dTomato by using the NahR/Psal promoter system on a pSB1C3 plasmid (See figure 1,2 and 3). The cells containing the plasmid were streaked on agar plates with different salicylate concentrations in the agar medium. Similarly, we also examined liquid cultures with different concentrations of salicylate in the solution. Both of these tests showed that the bacteria were producing the dTomato protein in presence of salicylate, independent of the salicylate being in liquid culture or in the solid growth medium. It can also be easily observed that the fluorescence increased with increasing salicylate concentrations (See figure 1 and 2). The measurements were made with the fluorometer that our team built in order to be able to characterize this system. These results were compared to MACS measurements on the same colonies and the measurements were correlated.
References
Nathan C Shaner, Robert E Campbell, Paul A Steinbach, Ben N G Giepmans, Amy E Palmer & Roger Y Tsien. “Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein”, 2004