Difference between revisions of "Part:BBa K1218026:Experience"
Vecchionis (Talk | contribs) (→Experimental data (provided by the 2013 Stanford-Brown iGEM Team)) |
Vecchionis (Talk | contribs) (→Experimental data (provided by the 2013 Stanford-Brown iGEM Team)) |
||
| Line 17: | Line 17: | ||
[[File:Star_Melt.jpg|center|475px]] | [[File:Star_Melt.jpg|center|475px]] | ||
''Figure 1:'' average of four trials on 32bp10CC Star sequence, P value of 10^-272 in paired T-Test. | ''Figure 1:'' average of four trials on 32bp10CC Star sequence, P value of 10^-272 in paired T-Test. | ||
| + | |||
| + | '''PAGE: electrical analysis of duplex formation''' | ||
| + | |||
| + | We utilized poly-acrylamide gel electrophoresis (PAGE)to produce visual representations of duplex formation, and we used this method to demonstrate that duplexing occurs as a 1:1 molar ratio between CC mismatches and silver ions is reached. | ||
| + | |||
| + | We tested undersaturation (<1:1 Ag:CC) and oversaturation (>1:1 Ag:CC) of the system, and showed the duplexing point. Duplexing became visually apparent at the 1.5:1 silver to mismatch ratio, which is slightly higher than expected. Nonetheless confirms both the role of silver in annealing mismatch-complimentary strands and that the set point of the system is near a 1:1 ratio. | ||
| + | |||
| + | [[File:PAGE.jpg|center|600px]] | ||
| + | ''Figure 3:'' 10% polyacrylamide gel in MOPS buffer, 32bp10CC strand with increasing molarity of silver (inset above lanes); duplexing begins to be visible at 1.5:1 silver to mismatch ratio-- while this is not ideal, it demonstrates visually the duplexing as it occurs; duplexes are the bands above the rest, indicative of the size difference that comes with duplexing. | ||
Revision as of 19:27, 27 September 2013
This experience page is provided so that any user may enter their experience using this part.
Please enter
how you used this part and how it worked out.
Experimental data (provided by the 2013 Stanford-Brown iGEM Team)
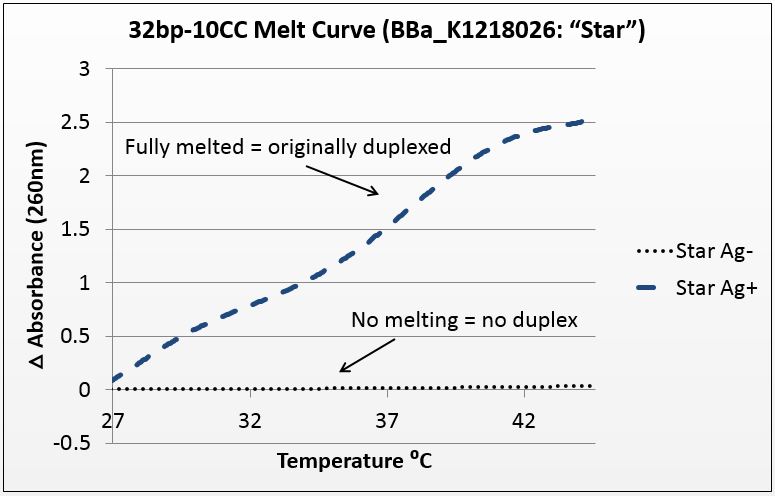
Thermal denaturation: thermodynamic evidence of silver binding
For this experiment, we sought to unequivocally prove that there was silver uptake by the excised duplex. To do this, we annealed the strands in two conditions: Ag+ (silver present) and Ag- (silver absent). The mismatch-complementary oligonucleotides in this sample were shown to only anneal in the presence of Ag+.
It is known that when double-stranded DNA is heated, it “melts,” or experiences a breakdown of its secondary structure. Beer’s law states that for a fixed volume and molecule, absorbance is proportional to concentration. As DNA melts, the concentration of nucleotides doubles, and the absorbance will increase similarly.
To show intercalation, we hypothesized that in this highly-mismatched strand, annealing will only occur in the presence of silver. Consequently, those strands should only show an absorbance increase with temperature if they were annealed with silver. That which does not anneal will not melt.
The graph below is the average of four trials, plotted for unit-less change in absorbance at 260nm (absorbance of dsDNA) over increasing temperature. The spectrophotometer we had access to restricted temperature to a maximum of 45°C, which we factored into the sequence design. 535 data points for each trial were taken across a 20°C temperature difference, rendering the ensuing data quite statistically significant.
Figure 1: average of four trials on 32bp10CC Star sequence, P value of 10^-272 in paired T-Test.
PAGE: electrical analysis of duplex formation
We utilized poly-acrylamide gel electrophoresis (PAGE)to produce visual representations of duplex formation, and we used this method to demonstrate that duplexing occurs as a 1:1 molar ratio between CC mismatches and silver ions is reached.
We tested undersaturation (<1:1 Ag:CC) and oversaturation (>1:1 Ag:CC) of the system, and showed the duplexing point. Duplexing became visually apparent at the 1.5:1 silver to mismatch ratio, which is slightly higher than expected. Nonetheless confirms both the role of silver in annealing mismatch-complimentary strands and that the set point of the system is near a 1:1 ratio.
Figure 3: 10% polyacrylamide gel in MOPS buffer, 32bp10CC strand with increasing molarity of silver (inset above lanes); duplexing begins to be visible at 1.5:1 silver to mismatch ratio-- while this is not ideal, it demonstrates visually the duplexing as it occurs; duplexes are the bands above the rest, indicative of the size difference that comes with duplexing.
Applications of BBa_K1218026
User Reviews
UNIQc3201ad400009345-partinfo-00000000-QINU UNIQc3201ad400009345-partinfo-00000001-QINU