Difference between revisions of "Part:BBa K115002"
| Line 77: | Line 77: | ||
2. A strain containing a vector with same backbone is used as control. Experimental groups and control groups are both cultured in 60mL LB-ampicillin (50 ng/µl) medium overnight at 37℃, 200rpm.<br> | 2. A strain containing a vector with same backbone is used as control. Experimental groups and control groups are both cultured in 60mL LB-ampicillin (50 ng/µl) medium overnight at 37℃, 200rpm.<br> | ||
3. Equally divide each group into two flasks, which is 30mL respectively. One of each group is cultured at 27℃, 200rpm and the other at 37℃, 200rpm.<br> | 3. Equally divide each group into two flasks, which is 30mL respectively. One of each group is cultured at 27℃, 200rpm and the other at 37℃, 200rpm.<br> | ||
| − | 4. Extract 5μl samples of each culture system every 6 hours. Diluted all of the samples to | + | 4. Extract 5μl samples of each culture system every 6 hours. Diluted all of the samples to 10^7 times and then spread them on solid LB-ampicillin (50 ng/µl) medium separately.<br> |
5. Count the number of colonies in 5 cm2 per plate after cultured for 24 hours at 37℃.<br> | 5. Count the number of colonies in 5 cm2 per plate after cultured for 24 hours at 37℃.<br> | ||
6. Three repicas are tested in each group.<br> | 6. Three repicas are tested in each group.<br> | ||
Revision as of 09:47, 16 October 2019
RNA thermometer (FourU)
An [http://2008.igem.org/Team:TUDelft/Temperature_analysis#RNA_thermometer RNA thermometer] that can be used for temperature sensitive post-transcriptional regulation which is based on a RNA thermometer from Salmonella Enterica Tyhpy that initiates translation at 37°C.
The image shows the secondary structure of the part after ligation to a protein coding part, as predicted by [http://rna.tbi.univie.ac.at/cgi-bin/RNAfold.cgi RNAfold]. Notice that the 3' end, including the scar and the start codon, does not belong to the part. The blue region is the Shine Dalgarno sequence which is the ribosome binding site. The blue nucleotides are the ones that had to be changed in order to get the desired secondary structure after introduction of the scar.
Free-living microorganisms are frequently exposed to changing environmental conditions. Nutrient availability, osmolarity, pH, temperature and many other parameters are being surveyed constantly. To avoid hazardous consequences, bacteria have established an intricate network of protective mechanisms. Often, regulation of environmentally controlled genes is realized at the transcriptional level by the action of regulatory proteins. However, several post-transcriptional, RNA-based strategies have been discovered recently. It is becoming increasingly clear that certain messenger RNAs (mRNAs) are not simply a substrate for ribosomes but contain control elements that modulate their own expression in a condition-dependent fashion. Structural changes in such sensory RNAs are induced by specific environmental changes. Two principally different classes can be distinguished: cis-acting RNA elements that bear their regulatory potential embedded within the mRNA sequence and trans-acting small, noncoding RNAs that function by base-pairing with complementary mRNA sequences encoded elsewhere in the genome. look more at [http://onlinelibrary.wiley.com/doi/10.1111/j.1574-6976.2005.004.x/full here] (edited by TCFSH_Taiwan)
2017 Team SVCE_CHENNAI's characterisation
We characterised the RNA thermometer(FourU) (BBa_K115002) part using GFP to show maximum fluroscence at 37ºC. The part works as expected and maximum level of fluorescence was observed at 37ºC.

The expression of GFP was found to be basal and minimum at 32ºC. A sudden induction in the expression was observed at the temperature 37ºC and it seemed to be gradually decreasing after 42ºC. This behaviour of RNA thermometer(FourU) observed supports the data given by other teams. Thus, the RNA thermometer(FourU) works as expected.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]

2017 Team SVCE_CHENNAI's characterisation
We characterised the RNA thermometer(FourU) (BBa_K115002) part using GFP to show maximum fluroscence at 37ºC. The part works as expected and maximum level of fluorescence was observed at 37ºC.

The expression of GFP was found to be basal and minimum at 32ºC. A sudden induction in the expression was observed at the temperature 37ºC and it seemed to be gradually decreasing after 42ºC. This behaviour of RNA thermometer(FourU) observed supports the data given by other teams. Thus, the RNA thermometer(FourU) works as expected. This is the adaptor

Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Characterized by BNU-China 2019
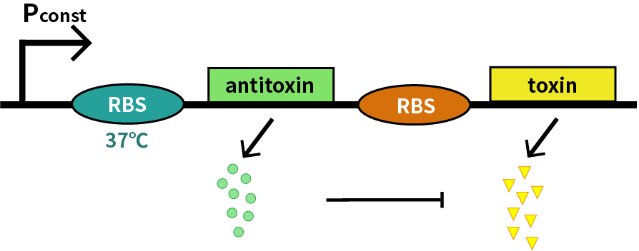
We characterize this RNA thermometer (FourU)(BBa_K115002) part by an antitoxin-toxin system, in which the downstream gene encodes for a constantly expressed toxin (relE: BBa_K185000) and the upstream gene encodes for a labile antitoxin (relB: BBa_K185048) under the control of the RNA thermometer (FourU). The expression of antitoxin counteracts lethality of toxin, which otherwise would cause death of bacteria. As a result, we can characterize the behavior of RNA thermometer (FourU) at different temperatures in a cell density-dependent manner in Escherichia coli K-12.
In order to measure the function of this RNA thermometer(FourU) at 27℃ and 37℃, we take E. coli introduced with a vector with the same backbone as control group.
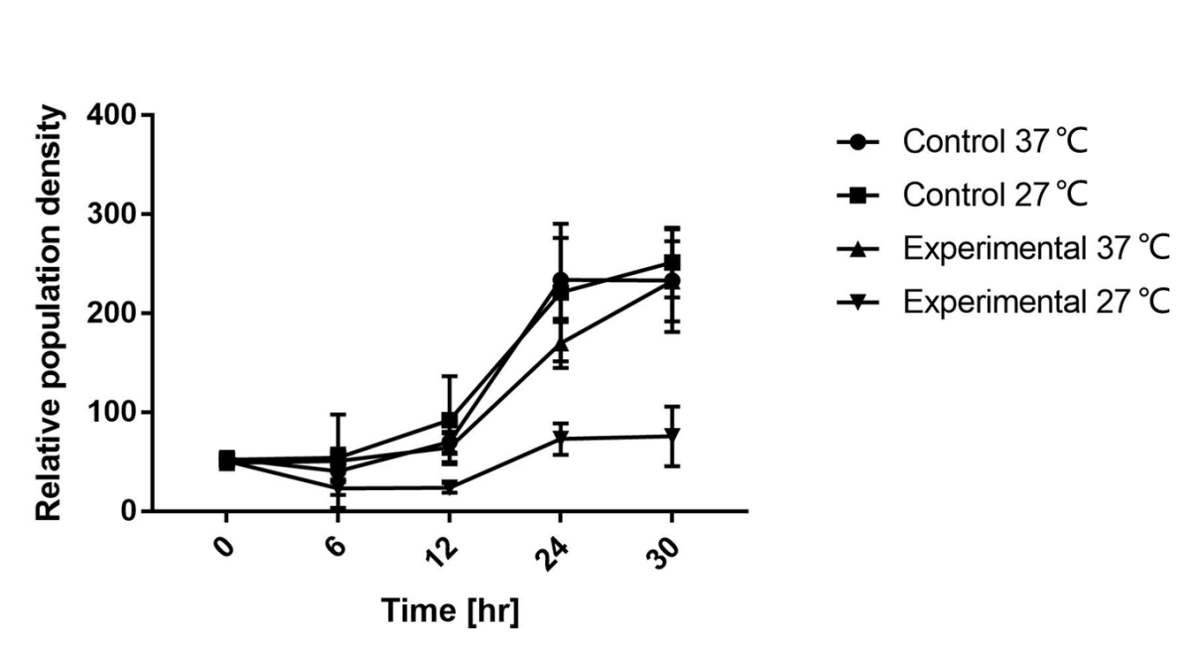
As is shown in Fig.1, there is nearly no difference of the relative population density between control and experimental groups at 37℃, which indicates the RNA thermometer (FourU) conducts expression of downstream gene. However, the population density of experimental group shows a significant decrease compared to control group at 27℃, caused by lack of antitoxin, which indicates the RNA thermometer (FourU) inhibits expression of antitoxin at 27℃.
With properties of the RNA thermometer (FourU), we construct a cold-triggered kill switch to prevent bacteria escaping from human body.
Experimental approach
1. Transform the plasmids into E. coli DH5α competent cells.
2. A strain containing a vector with same backbone is used as control. Experimental groups and control groups are both cultured in 60mL LB-ampicillin (50 ng/µl) medium overnight at 37℃, 200rpm.
3. Equally divide each group into two flasks, which is 30mL respectively. One of each group is cultured at 27℃, 200rpm and the other at 37℃, 200rpm.
4. Extract 5μl samples of each culture system every 6 hours. Diluted all of the samples to 10^7 times and then spread them on solid LB-ampicillin (50 ng/µl) medium separately.
5. Count the number of colonies in 5 cm2 per plate after cultured for 24 hours at 37℃.
6. Three repicas are tested in each group.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]