Difference between revisions of "Part:BBa K1033933:Experience"
(→Applications of BBa_K1033933) |
(→Applications of BBa_K1033933) |
||
| Line 9: | Line 9: | ||
2019 iGEM team Linkoping Sweden validated this part.<br><br> | 2019 iGEM team Linkoping Sweden validated this part.<br><br> | ||
| − | + | [[File:T--Linkoping_Sweden--pinkwhite156.jpeg|700px|thumb|center|<b>Figure 2.</b> E. coli (BL21) cells used for pink-white screening, the cells were incubated for 16 hours in 37 degrees Celsius. <partinfo>BBa_K3182100</partinfo> was cut with BamHI and PstI to remove pCons-AsPink and <partinfo>BBa_K3182006</partinfo> (magainin 2) and <partinfo>BBa_K3182104</partinfo> (CHAP) was ligated into the plasmid. The white colonies indicate a successful ligation. All the colonies that were later colony screened with PCR amplification of the insert and the ampified strand was run on an agarose gel. The gel implied that all screened colonies was successful, i.e. contained <partinfo>BBa_K3182100</partinfo> with magainin 2 / CHAP instead of pCons-AsPink. ]] | |
<br><br> | <br><br> | ||
Revision as of 08:45, 27 August 2019
This experience page is provided so that any user may enter their experience using this part.
Please enter
how you used this part and how it worked out.
Applications of BBa_K1033933
2019 iGEM team Linkoping Sweden
2019 iGEM team Linkoping Sweden validated this part.
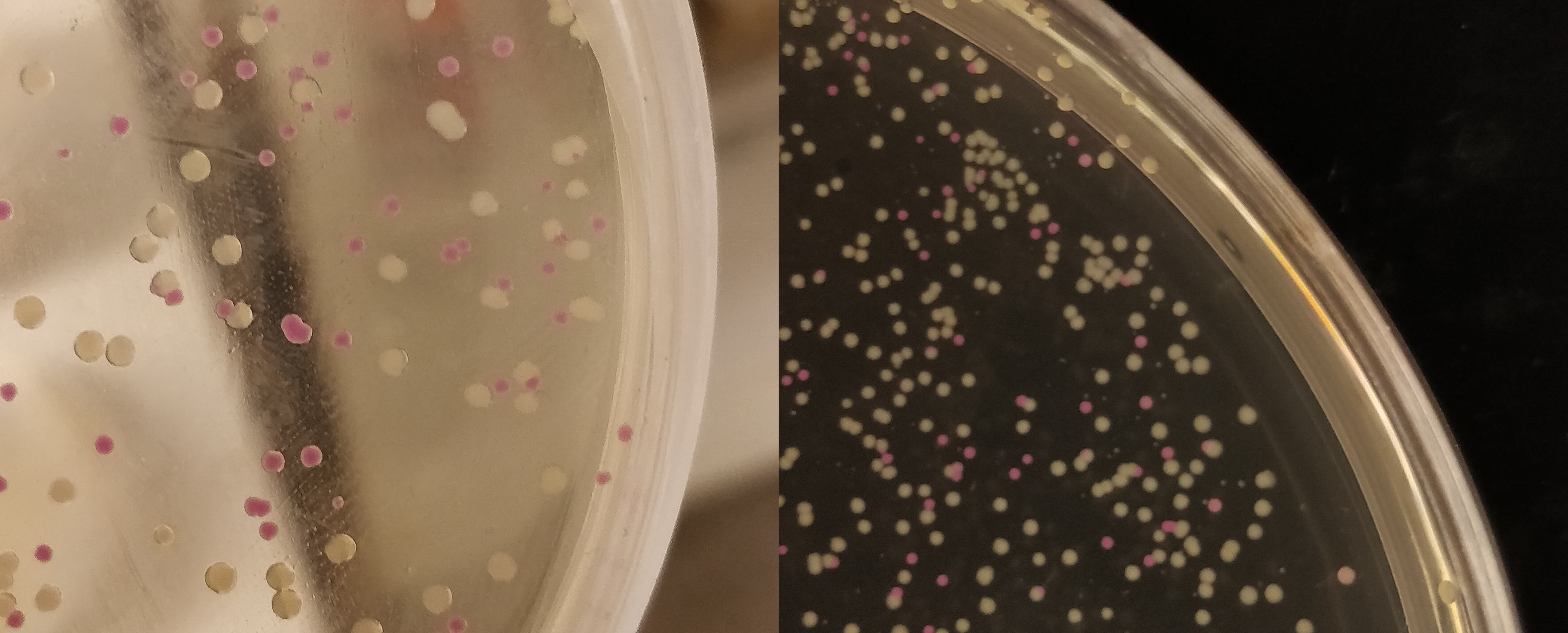
Figure 2. E. coli (BL21) cells used for pink-white screening, the cells were incubated for 16 hours in 37 degrees Celsius. BBa_K3182100 was cut with BamHI and PstI to remove pCons-AsPink and BBa_K3182006 (magainin 2) and BBa_K3182104 (CHAP) was ligated into the plasmid. The white colonies indicate a successful ligation. All the colonies that were later colony screened with PCR amplification of the insert and the ampified strand was run on an agarose gel. The gel implied that all screened colonies was successful, i.e. contained BBa_K3182100 with magainin 2 / CHAP instead of pCons-AsPink.
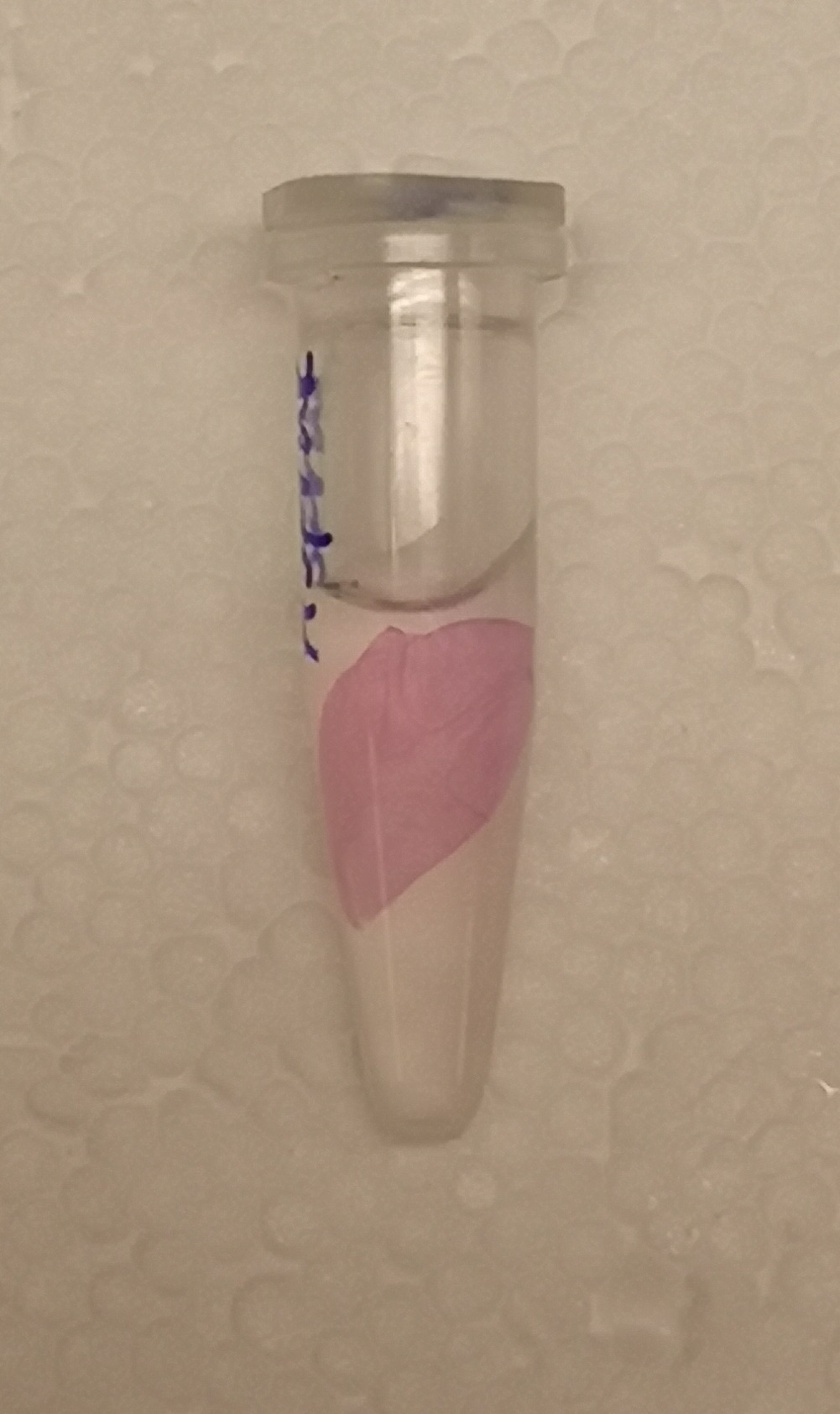
CBD-asPink bindning capacity
To test the bindning capacity of CBD-asPink (BBa_K3182000) the white microbial cellulose bandage was into the sonicated lysate of BL21(DE3) with the expressed fusion protein (see Figure 1) and incubated for 30 minutes. The bandage was then washed thrice in 70% ethanol which confirmed that the bindning of CDB-asPink was still intact after the washes to the bandage.
CBD-asPink thrombin cleavage
User Reviews
UNIQ285d4a7904159e36-partinfo-00000005-QINU UNIQ285d4a7904159e36-partinfo-00000006-QINU