Difference between revisions of "Part:BBa I0500:Experience"
(→User Reviews) |
|||
| Line 46: | Line 46: | ||
<I><b>[http://2015.igem.org/Team: HKUST-Rice 2015]</B></I> | <I><b>[http://2015.igem.org/Team: HKUST-Rice 2015]</B></I> | ||
|width='60%' valign='top'| | |width='60%' valign='top'| | ||
| − | [http://2015.igem.org/Team:HKUST-Rice HKUST-Rice Team] characterised [https://parts.igem.org/Part:BBa_I0500 BBa_I0500] by ligating with [https://parts.igem.org/Part:BBa_I13504 BBa_I13504] (a GFP reporter) in low copy plasmid pSB3K3. We aimed to obtain a dose response curve capturing the dynamic range and maximum induction concentration inorder to compare its behaviour when parallelly expressed with another sensor. | + | [http://2015.igem.org/Team:HKUST-Rice HKUST-Rice Team] characterised [https://parts.igem.org/Part:BBa_I0500 BBa_I0500] by ligating with [https://parts.igem.org/Part:BBa_I13504 BBa_I13504] (a GFP reporter) in low copy plasmid pSB3K3. We aimed to obtain a dose response curve capturing the dynamic range and maximum induction concentration inorder to compare its behaviour when [https://parts.igem.org/Part:BBa_K1682017 parallelly expressed with another sensor]. |
[[Image:HKUST_RICE2015Pbad.jpg|centre|thumb|400px| Dose responses of cells carrying [https://parts.igem.org/Part:BBa_I13540 BBa_I13540] in pSB3K3 under increasing L-Arabinose concentration. Error bars, s.d (n=3) ]] | [[Image:HKUST_RICE2015Pbad.jpg|centre|thumb|400px| Dose responses of cells carrying [https://parts.igem.org/Part:BBa_I13540 BBa_I13540] in pSB3K3 under increasing L-Arabinose concentration. Error bars, s.d (n=3) ]] | ||
| − | Characterisation was done using strain DH10B. | + | Characterisation was done using <i>E. coli</i> strain DH10B. Overnight induced cell cultures were diluted(20x) in pre-warmed M9 medium and induced with corresponding L-Arabinose concentrations the next day. Measurements for population-averaged assay (Fluorescence/OD600) were taken after 5.5 hours of induction and incubation at 37degC when the OD600 was around 0.3-0.4. |
|} | |} | ||
Revision as of 02:06, 16 September 2015
This experience page is provided so that any user may enter their experience using this part.
Please enter
how you used this part and how it worked out.
Applications of BBa_I0500
- When grown with 0.2% arabinose, promoter is weak-medium. [jb, 5/24/04] Part may not be compatible with MC4100 as cell line is araD 139.
- MC4100 is not a good chassis for operating BBa_I0500 (pBad promoter). The feed-forward regulation of the endogenous promoter controlling expression of the arabinose transporter prevents linear induction with increasing arabinose concentration. (Engineered strain from Keasling's lab, used by jrk for operation of the screening plasmid.) [cconboy 04]
- Observations of induced expression of GFP (BBa_E0840) are consistent with previous comments about weak promoter signal induction by jb 5/24/04. [melissali, Berkeley iGEM 2005]
PC and AraC are located on the complementary strand, reading right to left as written.
- At least one registry stock contains a deletion of the C at base 1194. This is after the transcriptional start but before the translation start, so it may not be significant. Parts with this mutation have been qualitatively observed to function normally.
- Can with success be combined with a promoter from pBAD SPL to obtain a very low leakiness and an appropriate strength. This is very efficient for expressing lethal proteins.
User Reviews
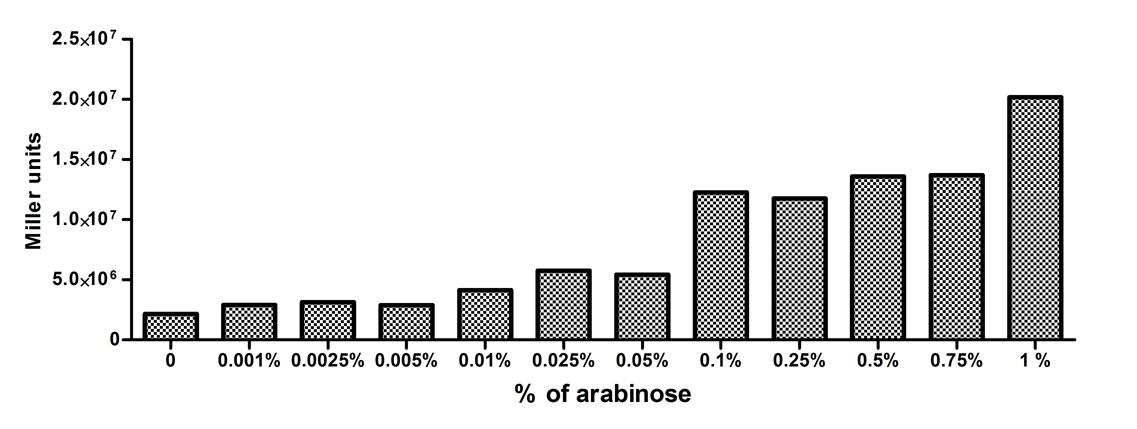
[http://2010.igem.org/Team:Slovenia 2010 iGEM team Slovenia] further characterized the pBAD/araC promoter (Part:Bba_I0500) using lacZ gene that encodes for beta-galactosidaze enzyme (Part:Bba_K323133). pBAD promoter is an arabinose inducible promoter. When incubating E.coli cultures containing the lacZ under pBAD/araC promoter, increasing concentrations of arabinose leads to higer promoter activity that results in higher amounts and subsequently higer activity of beta-galactosidase enzyme as seen from figure below. The resulting graph shows a trend of increasing beta-galactosidase activity over increasing concentrations of arabinose.
The tested cultures incubated at different concentrations of arabinose (i.e. 0%, 0.001%, 0.0025%, 0.005%, 0.01%, 0.025%, 0.05%, 0.1%, 0.25%, 0.5%, 0.75% and 1%) have shown a trend of increasing beta-galactosidase activity with raising arabinose concentration. The activity was highest at 1% added arabinose, which implies the promotor should be induced by this arabinose concentration for reaching maximum expression of the regulated protein. However, some beta-galactosidase activity was observed even at 0% added arabinose, which indicates slight leakage of the pBAD promoter.
pBAD/araC_lacZ_DTER construct (Part:Bba_K323133) includes a beta-galactosidase (lacZ) gene, the expression of which is regulated by the pBAD promoter (Part:Bba_I0500). This construct was designed for the sole purpose of characterising the pBAD promoter with the beta-galactosidase assay (for a detailed description of the method see the 2010 iGEM team Slovenia wiki).
UNIQ636baf192b9c991e-partinfo-00000000-QINU
|
•••••
[http://2015.igem.org/Team: HKUST-Rice 2015] |
[http://2015.igem.org/Team:HKUST-Rice HKUST-Rice Team] characterised BBa_I0500 by ligating with BBa_I13504 (a GFP reporter) in low copy plasmid pSB3K3. We aimed to obtain a dose response curve capturing the dynamic range and maximum induction concentration inorder to compare its behaviour when parallelly expressed with another sensor. 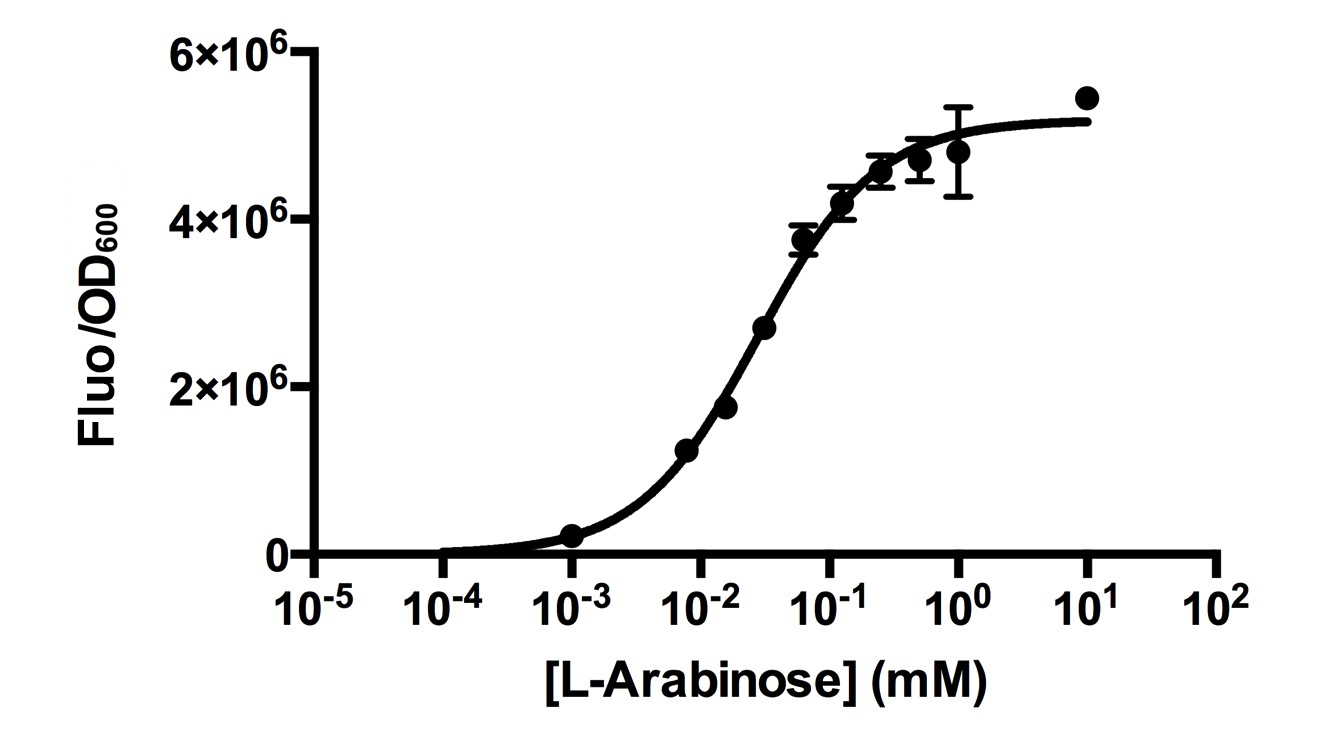 Dose responses of cells carrying BBa_I13540 in pSB3K3 under increasing L-Arabinose concentration. Error bars, s.d (n=3)
|
|
•••••
Antiquity |
This review comes from the old result system and indicates that this part worked in some test. |
|
[http://2012.igem.org/Team:UNITN-Trento Trento 2012] |
We were unable to extract this part from the distribution kit, so we developed our own! Please head to BBa_K731201 for documentation and characterization. |
|
iGEM Groningen 2009 |
The sequence was listed as inconsistent, and the ligations of parts behind the promoter failed. Restriction of isolated plasmids showed fragments of unexpected sizes. |
|
•••••
[http://2011.igem.org/Team:Groningen 2011 iGEM team Groningen] |
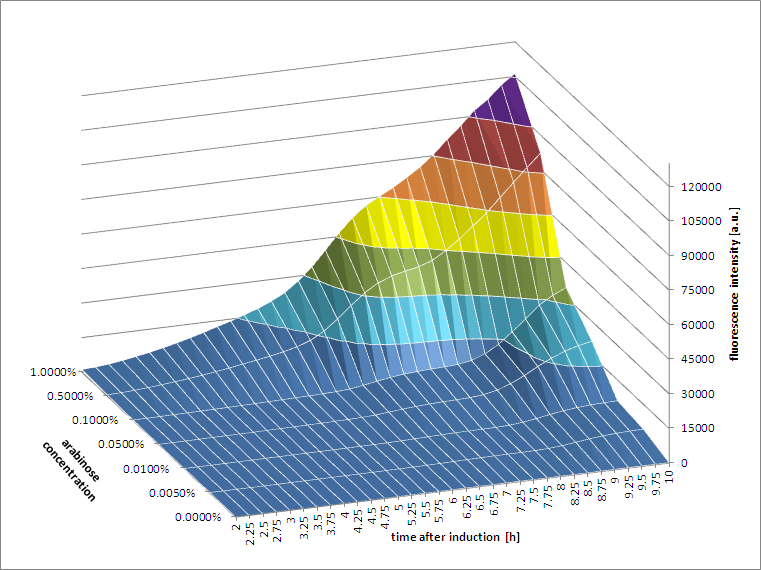
[http://2011.igem.org/Team:Groningen 2011 iGEM team Groningen] characterized the pBAD/AraC promoter (Part:Bba_I0500) even further using GFP and measuring the fluorescence in a fluorimeter over time, utilizing different arabinose concentrations to induce the promoter. After analysis of the obtained data (see graphs below), it is clear that the induction is faster and stronger with higher arabinose concentrations. It shows a dynamic response over a range of arabinose concentrations and over time. Because of that it can be treated as a precise tool capable of giving different output levels. The measurements were done in DH5alpha strain carrying a pSB1C3 with BBa_K607036 in triplicate.
|
|
•••••
[http://2011.igem.org/Team:Cambridge Cambridge 2011] |
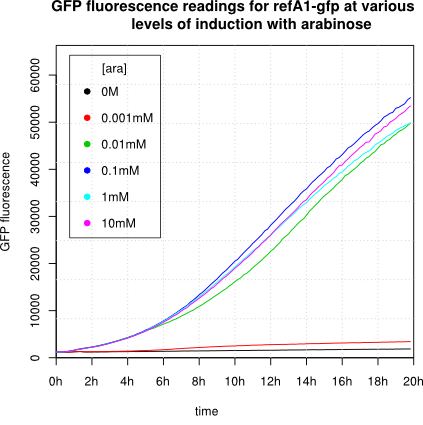
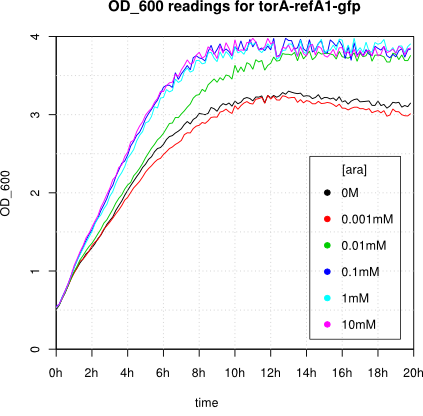
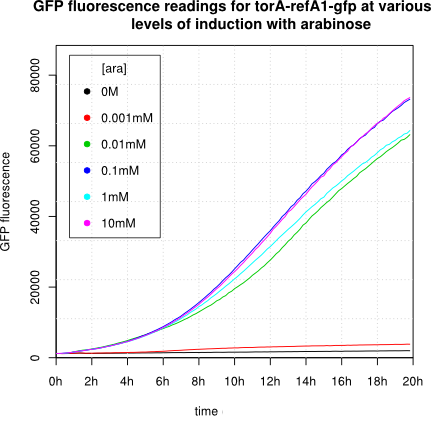
We observed an 'all-or-nothing' response in our use of the pBAD promoter. We grew up cells of the strain [http://cgsc.biology.yale.edu/Strain.php?ID=111773 BW27783] overnight at 37°C. We then diluted the culture 10-fold and grew the cells back up to an OD_600 of 0.5 to catch the cells in mid-exponential phase. We then induced with arabinose at various concentrations from 0.001mM up to 10mM and took OD and GFP readings on a plate reader over the next 20 hours. During this time the cells were held at 37°rees;C. The cells contained our ReflectinA1-sfGFP and TorA-ReflectinA1-sfGFP constructs on pSB3K3 plasmids. This work was not intended to characterise the pBAD promoter, but it highlights some of the difficulties in doing so:
Note: 1mM arabinose corresponds to 0.015% w/v.
|
|
••••
[http://2014.igem.org/Team: Hong_Kong_HKUST 2014] |
2014 HKUST iGEM team previously submitted characterization of Part BBa_I0500 that described an all-or-none behavior of the promoter. Yet, the conclusion needs to be revised due to inconsistency in subsequent repeats. We shall upload our latest data once our results are ready.} UNIQ636baf192b9c991e-partinfo-0000000A-QINU |