Part:BBa_K4275041
pT7-RBS-CipA1B2C-tT7
Strong promoter and terminator are used for high production.The protein produced has the ability to interact with 2 enzymatic subunits that are fused with a type I dockerin because the primary scaffoldin contains 2 type I cohesin domain. The cohesin-dockerin interaction fixes the subunits onto the primary scaffold allowing the enzymes to work synergetically to perform a cascade of catalytical reactions. One CBM ensures the cellulose or PET in the surrounding are attracted to the domains and allows them to be digested.
Usage and Biology
The protein scaffold of the mimic primary scaffoldin component contains 2 type I cohesin domain that interacts with the type I dockerin domain fused on other fusion protein to fix them onto the scaffold as a complex, the CBM domain on the protein attracts PET and cellulose in the surrounding, generating close proximity between the fixed enzyme on the scaffold and the substrate in the case of cellulose and PET degradation[1], for the catalytical reactions to happen faster. The primary scaffold can also be assembled to the secondary scaffold as it contains a type II dockerin domain to construct larger cellulosome-like complexes. And the paired strong promoter and terminator grants efficient production of the protein.
Characterisation of the Cellulosome Complex
Mini-scaffold construction of cellulosome
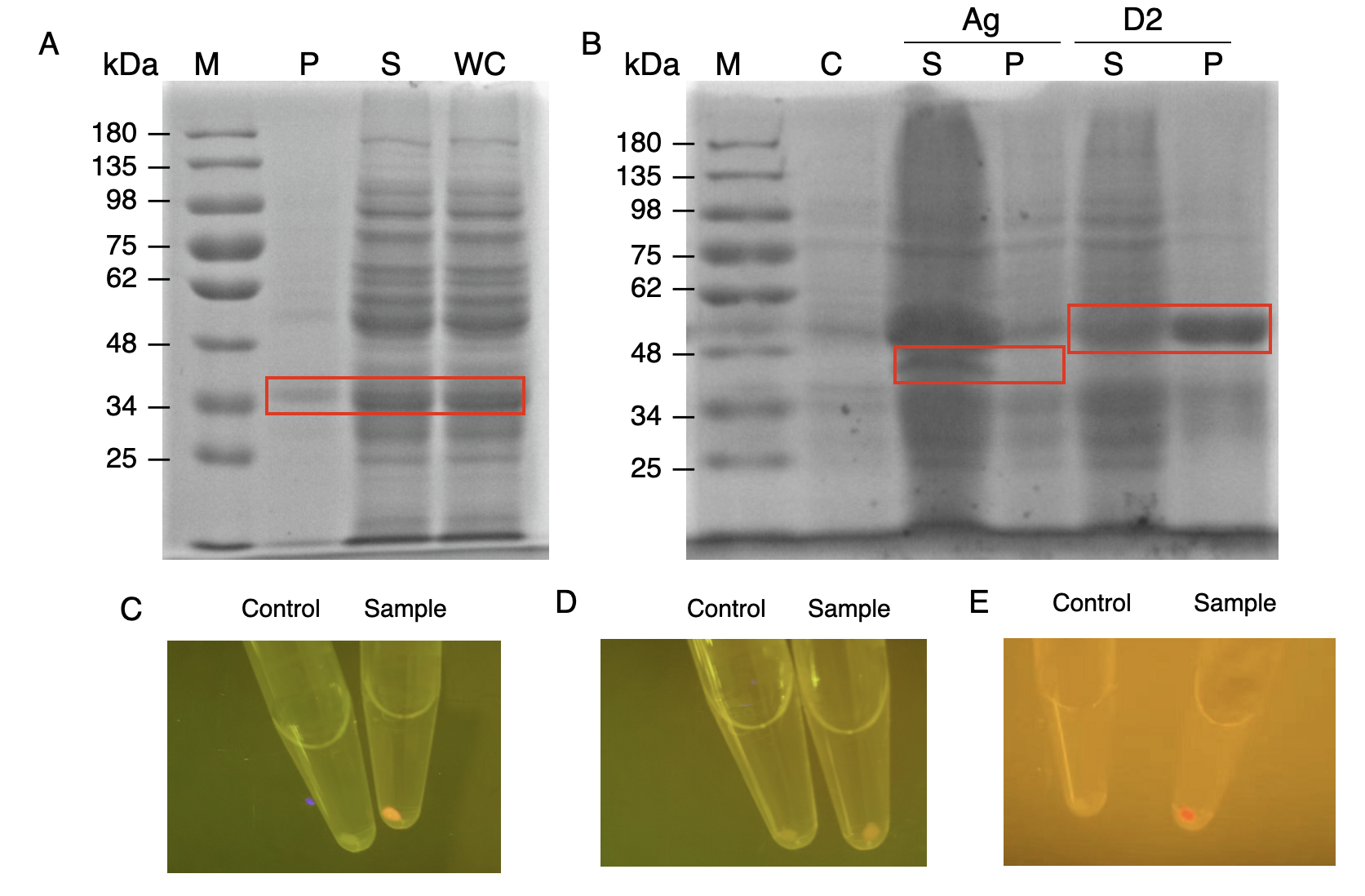
We constructed E.coli expression vectors for the mini-scaffold protein subunits. The scaffoldin components of the wild-type cellulosome subunits are large protein scaffolds that would bring a massive protein burden to the bacterial host secreting them. We modified the coding sequences for the wild-type cellulosome protein scaffold, as shown in (Fig.3A and Fig.3B) The mini-scaffolds were successfully expressed by our host, verified by the SDS-PAGE analysis shown in (Fig.3C and Fig.3D)
Functionality testing of our mini-scaffold
In order to verify the three levels of protein-protein interaction that assembles our cellulosome complex, Ag3-eforRED, DocI-eforRED, and DocII-eforRED vectors were constructed and cultured for IPTG-inducible expression (Fig. 3D). SDS-page analysis was performed with lysed cells and all three targeted proteins were identified in both whole cell and supernatant (Fig. 4A and 4B).
The nanobody-antigen interaction was verified by mixing intact E. coli cells displaying Neae-Nb3 with the supernatant of Ag3-eforRED (Fig. 3A). Red fluorescent characteristics were observed in the pellets after resuspending the centrifuged mixture, which is absent in the control group that only contains Neae-Nb3 (Fig. 4C).
After that, the type II cohesin-dockerin interaction was tested using the mixture of Neae-Nb3, OlpB-Ag3, and the type II dokerin fused with eforRED (Fig. 3B). A negative control lacking OlpB-Ag3 was set up for result comparison. Centrifugation was used to remove supernatant and the red fluorescence was only identified in pellets of the sample group, confirming the type II cohesin-dockerin interaction (Fig. 4D).
Finally, the association between type I cohesin and type I dockerin was validated using the mixture of Neae-Nb3, OlpB-Ag3, CipA1B2C, and DocI-eforRED (Fig. 3C), red fluorescence was detected in the resuspended mixture while it was not observed in the control group lacking the primary scaffold CipA1B2C (Fig. 4E), verifying the type I cohesin-dockerin interaction.
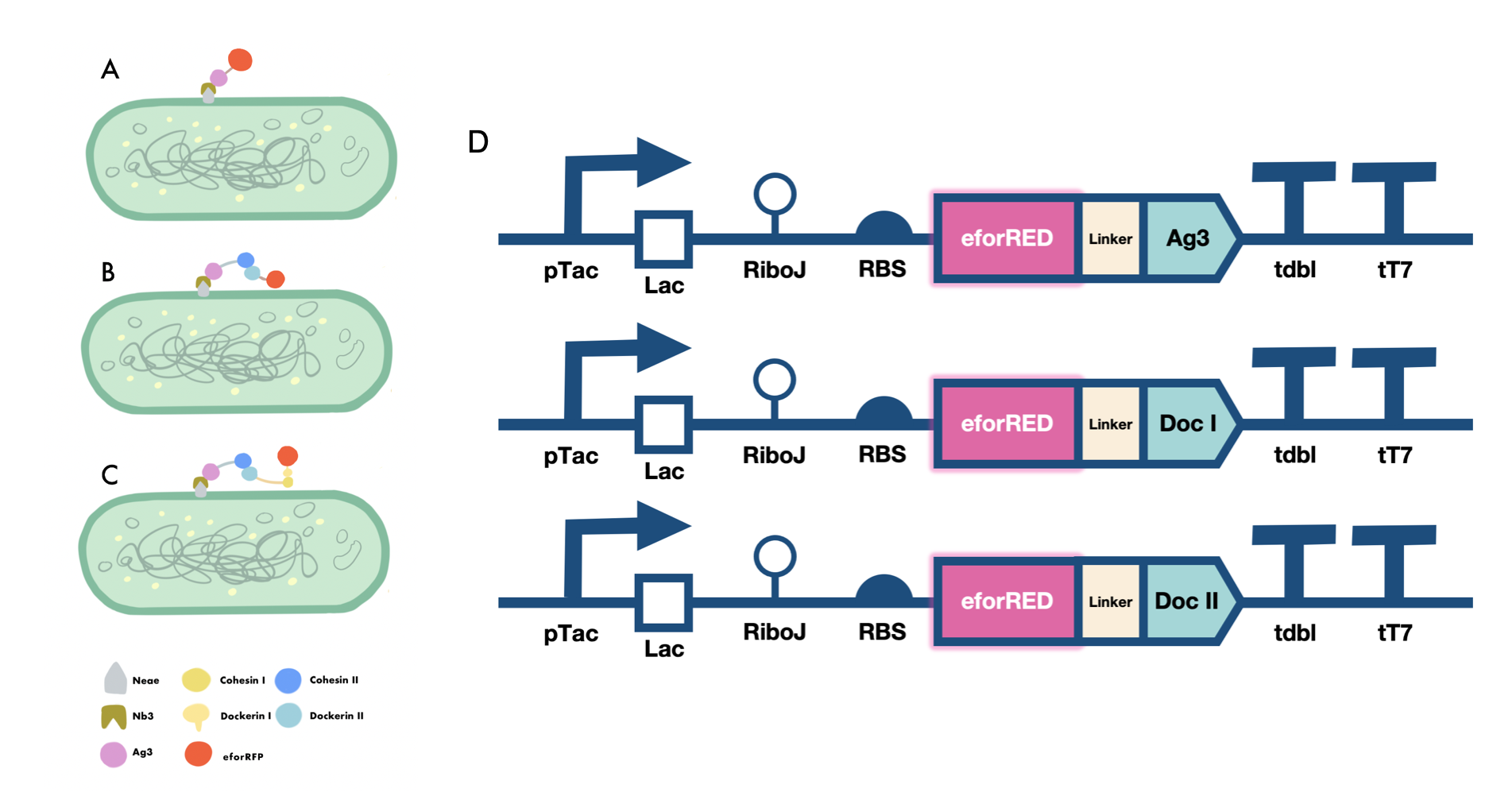
Cellulosome construction
We assembled the cellulose-like complex on the surface of E.coli by adding primary scaffold proteins, cellulases and cellulase boosters onto E.coli expressing secondary scaffold proteins. The mixture was centrifuged and resuspended in tris-HCl. The mixture underwent centrifugation and resuspension using tris-HCl, and cellulose was added to the mixture.
After 24h, the mixture was filtered and tested for glucose by Benedict's test. From the result, we determined that the cellulosome-like complexes are able to degrade cellulose at a higher efficiency than cell-free cellulases mixture. The overall success in engineering our project was verified by the successful construction of cellulosome complex and degrading cellulose to reducing sugars.

Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 2073
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 354
Illegal XhoI site found at 1277 - 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 583
Illegal AgeI site found at 365
Illegal AgeI site found at 709
Illegal AgeI site found at 908
Illegal AgeI site found at 1579 - 1000COMPATIBLE WITH RFC[1000]
References
1. Anandharaj, Marimuthu et al. "Constructing A Yeast To Express The Largest Cellulosome Complex On The Cell Surface". Proceedings Of The National Academy Of Sciences, vol 117, no. 5, 2020, pp. 2385-2394. Proceedings Of The National Academy Of Sciences, https://doi.org/10.1073/pnas.1916529117.
| None |

