Part:BBa_K4090004
Silicatein
Introduction=
Silicatein is a protein from the demosponge. It could induce the precipitation of the silica with the presence of silicic acid or TMOS. There are several kinds of silicateins, and the biobrick below is the short version of silicatein-alpha gene after the codon optimization base on the codon bias of E.coli.
Usage and Viability
To test the ability of silicatein in inducing precipitation of silica, it needs to be placed on the cell surface. As a result, in order to expose the silicatein to the favorable environment, it is co-expressed with the INP, a kind of transmembrane protein which could bring the protein to the cell surface.
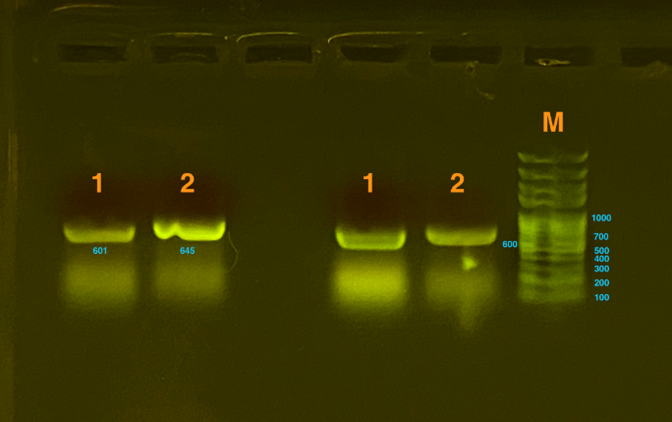
Gibson Assembly was used to transform the gene. Then, the gel electrophoresis was used to test whether plasmids were constructed successfully. Since the gene that inserted is too long to detect as a whole, which is about 2000bp, our team decided to detect sequences of two connections (one connected the silicatein with the vector and the other connected the INP with silicatein) , both of which are approximately 600bp. From the graph below, we could see that the result was positive, proving that the plasmids are successfully constructed.
Fig.2 represents the results of gel electrophoresis to test the fragment after Gibson Assembly. The length is about 600bp, which, together with the results from sequencing, indicates a positive outcome.
After using the Gibson Assembly to transform the INP-silicatein gene in the E.coli and cultured them under appropriate environment, controlled experiment is performed. In order to know whether the silicatein was also expressed inside the cell, part of the E.coli were homogenized by ultrasonic comminution and added to the sample. The graph below showed that the experimental groups present higher OD values, and the group with homogenization has higher OD values than the group without homogenization. The result shows that silicatein could induce the precipitation of silica effectively, though some of them are expressed inside the cell.
Fig.3 represents qualitative OD values, showing that there is sufficient amount of silicatein produced, since:
•Both NC Control are darker (NC1 remain as yellow which indicates that it needs MORE NaOH to turn blue, it contains more acids).
•Both silicatein mixture (with / without homogenization) have higher OD810nm, meaning that there are more bacteria inside the test tube (the solution is thicker / not as clear as NCs).
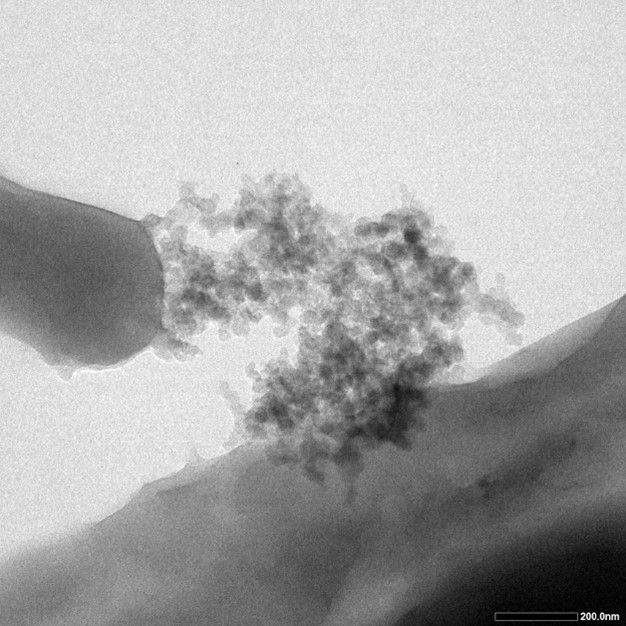
TEM testing is also utilized in order to offer a direct proof of the silicatein’ s function. Five drips of bacteria mixture of different concentration were added onto the five pieces of carbon film, and were put into the 80 degree Celsius dryer. After they are completely dry, TEM testing was carried out. The dark particles on the surface of cells, which are the silcia particles, further verified that the INP could bring the protein to the cell surface.
Fig.4 shows the results from TEM testing. Dark proportions represent silicon dioxide produced on the surface of cells.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 664
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
References
[1] Hale, L. V., Ma, Y. F. & Santerre, R. F. Semi-quantitative fuorescence analysis of calcein binding as a measurement of in vitro mineralization. Calcif. Tissue Int. 67, 80-84(2000). Ramachandran S K.
[2] Dick J, Windt W D, Graef B D, et al. Bio-deposition of a calcium carbonate layer on degraded limestone by Bacillus species[J]. Biodegradation, 2016(4): 357-367.
[3] Qian C X, Chen H C, Ren L F, et al. Self-healing of early age cracks in cement-based materials by mineralization of carbonic anhydrase microorganism[J]. Frontiers in Microbiology, 2015(6): 1-9.
| None |