Part:BBa_K3016600
YGFP, a slow bleaching spectral variant of GFP
This part contains the CDS of YGFP, a slow bleaching variant of GFP.
Biology
YGFP is a slow bleaching GFP variant derived from a GFP mutant, with different spectral characteristics to regular GFP and YFP. YGFP has an excitation maximum at around 500 nm, and emission maximum at around 510 nm. It is not excited at or over 525 nm, which makes it usable in combination with longer wavelength excited fluorescent proteins. YGFP also retains the fluorescence longer compared to e.g. YFP. (Hansen & Atlung, 2011)
Characterization
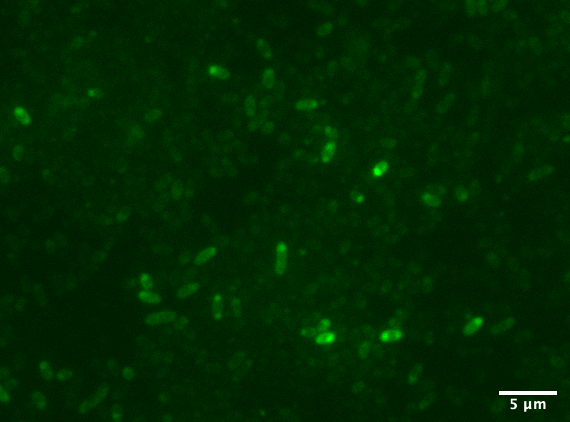
Aalto-Helsinki characterized this part by fusing it with Vibrio natriegens' TorA Tat signal peptide (BBa_K3016100), creating a composite TorA-YGFP part (BBa_K3016200), to test its functionality in periplasmic protein localisation studies in V. natriegens and Escherichia coli DH5a.
In the images above we see Vibrio natriegens and Escherichia coli DH5a cells expressing TorA-YGFP, which clearly fluoresces in both bacteria. Note the polar localisation of fluorescence. This can be a sign of periplasmic localisation of YGFP under osmotic pressure (Sochacki et al., 2011) or inclusion body formation (Jong et al., 2017).
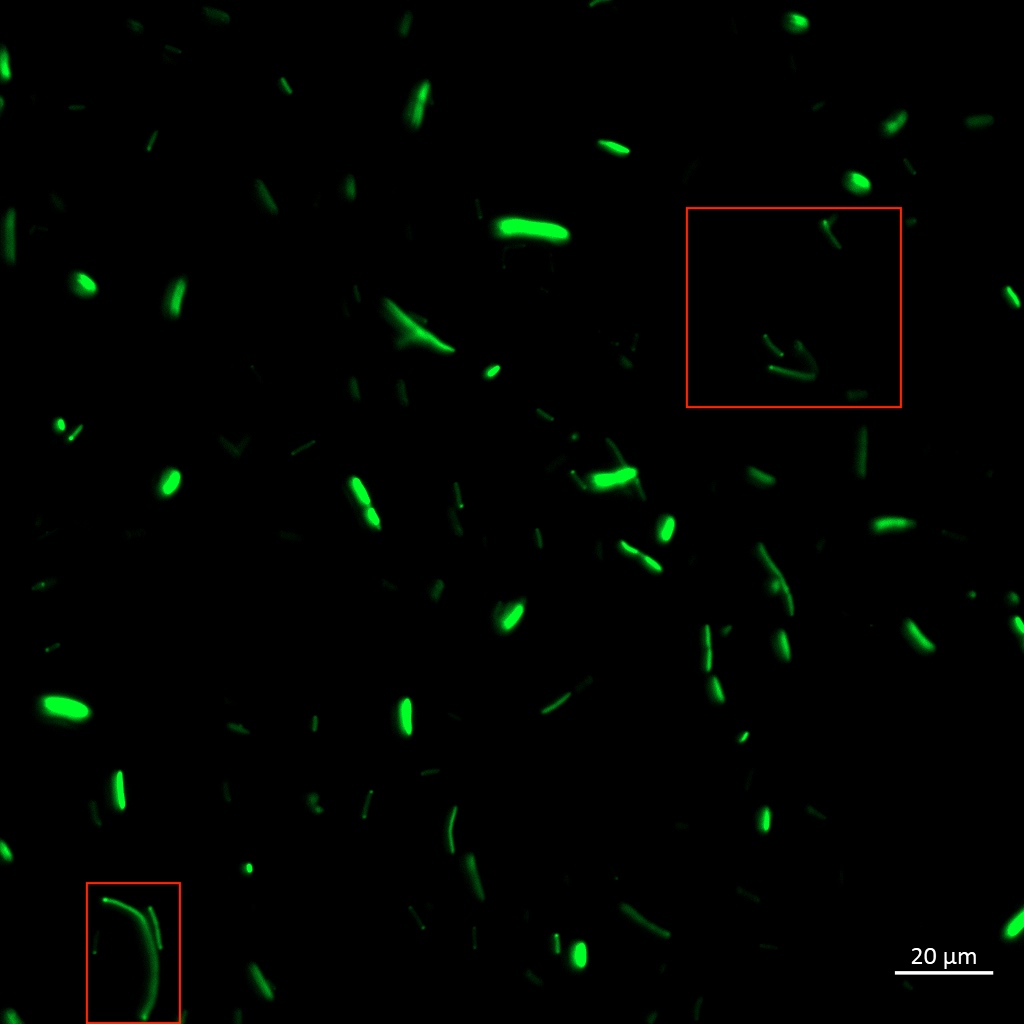
To be certain of successful periplasmic translocation, a cell fractionation experiment was performed on Vibrio natriegens. The periplasmic fraction of TorA-YGFP expressing cells was extracted ([ protocol]) and placed under UV light, image below. The presence of YGFP in the periplasmic fractions can be seen clearly.
These results indicate that the YGFP protein is active in both Vibrio natriegens' and Escherichia coli DH5a, and that it can successfully translocate into the periplasm of Vibrio natriegens, and possibly even of Escherichia coli, via the Tat pathway.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 679
- 1000COMPATIBLE WITH RFC[1000]
References:
Alanen, H. I., Walker, K. L., Suberbie, M. L. V., Matos, C. F., Bönisch, S., Freedman, R. B., ... & Robinson, C. (2015). Efficient export of human growth hormone, interferon α2b and antibody fragments to the periplasm by the Escherichia coli Tat pathway in the absence of prior disulfide bond formation. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research, 1853(3), 756-763.
Jong, W. S., Vikström, D., Houben, D., de Gier, J. W., & Luirink, J. (2017). Application of an E. coli signal sequence as a versatile inclusion body tag. Microbial cell factories, 16(1), 50.
Sochacki, K. A., Shkel, I. A., Record, M. T., & Weisshaar, J. C. (2011). Protein diffusion in the periplasm of E. coli under osmotic stress. Biophysical journal, 100(1), 22-31.
Hansen, F. G., & Atlung, T. (2011). YGFP: a spectral variant of GFP. BioTechniques, 50(6), 411-412. https://doi.org/10.2144/000113691
//cds/reporter/gfp
//function/reporter/fluorescence
| biology | fuorescence |
| protein | YGFP |