Difference between revisions of "Part:BBa K404167"
| Line 2: | Line 2: | ||
<partinfo>BBa_K404167 short</partinfo> | <partinfo>BBa_K404167 short</partinfo> | ||
<br><b>Construct containing the mVenus N-terminal fusion to VP2, a NLS & VP1up insertion and the Biotinylation Acceptor peptide motif in the 587 loop</b> | <br><b>Construct containing the mVenus N-terminal fusion to VP2, a NLS & VP1up insertion and the Biotinylation Acceptor peptide motif in the 587 loop</b> | ||
| + | {| style="color:black" cellpadding="6" cellspacing="1" border="2" align="center" | ||
| + | ! colspan="2" style="background:#66bbff;"|[https://parts.igem.org/Part:BBa_K404204 ViralBrick-453-Z34C)] | ||
| + | |- | ||
| + | |'''BioBrick Nr.''' | ||
| + | |[https://parts.igem.org/Part:BBa_K404167 BBa_K404167] | ||
| + | |- | ||
| + | |'''RFC standard''' | ||
| + | |[https://parts.igem.org/Help:Assembly_standard_10 RFC 10] | ||
| + | |- | ||
| + | |'''Requirement''' | ||
| + | |pSB1C3<br> | ||
| + | |- | ||
| + | |'''Source''' | ||
| + | | | ||
| + | |- | ||
| + | |'''Submitted by''' | ||
| + | |[http://2010.igem.org/Team:Freiburg_Bioware FreiGEM 2010] | ||
| + | |} | ||
| + | |||
<h2>CMV</h2> | <h2>CMV</h2> | ||
Revision as of 03:08, 28 October 2010
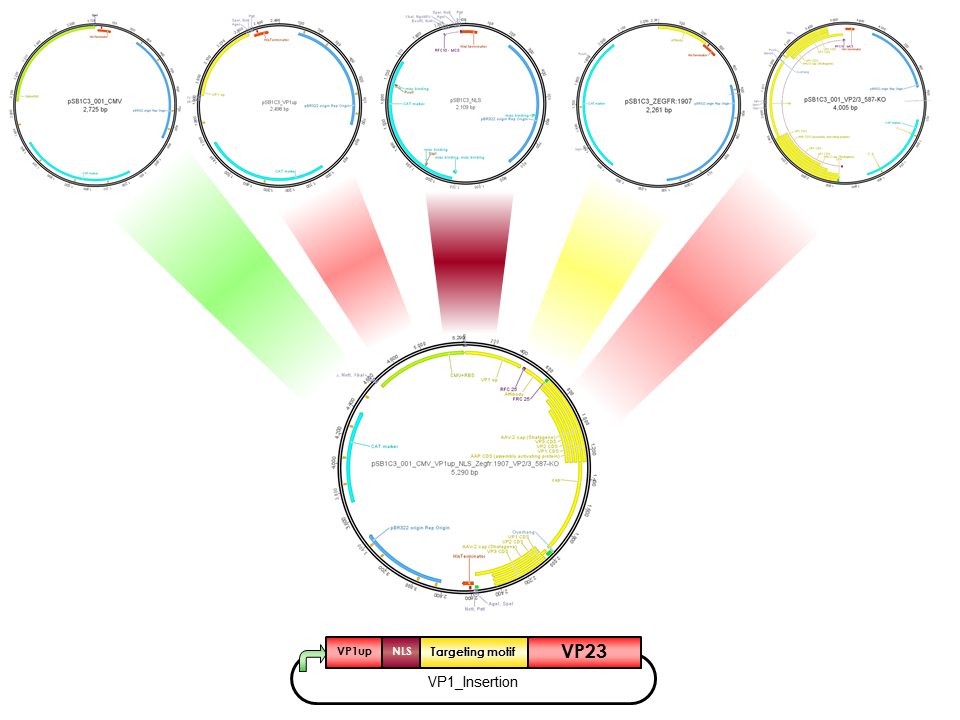
pCMV_VP1up_NLS_mVenus_VP23(ViralBrick-587-KO-BAP)
Construct containing the mVenus N-terminal fusion to VP2, a NLS & VP1up insertion and the Biotinylation Acceptor peptide motif in the 587 loop
| ViralBrick-453-Z34C) | |
|---|---|
| BioBrick Nr. | BBa_K404167 |
| RFC standard | RFC 10 |
| Requirement | pSB1C3 |
| Source | |
| Submitted by | [http://2010.igem.org/Team:Freiburg_Bioware FreiGEM 2010] |
CMV
CMV promoter is derived from human Cytomegalovirus, which belongs to Herpesvirus group. All family members share the ability to remain in latent stage in the human body. CMV is located upstream of immediate-early gene. However, CMV promoter is an example of widely used promoters and is present in mammalian expression vectors. The advantage of CMV is the high-level constitutive expression in mostly all human tissues [Fitzsimons et al., 2002].
Capsid
(BBa_K404006)
The AAV capsid consists of 60 capsid protein subunits composed of the three cap proteins VP1, VP2, and VP3, which are encoded in an overlapping reading frame. Arranged in a stoichiometric ratio of 1:1:10, they form an icosahedral symmetry. The mRNA encoding for the cap proteins is transcribed from p40 and alternative spliced to minor and major products. Alternative splicing and translation initiation of VP2 at a nonconventional ACG initiation codon promote the expression of the VP proteins. VP1, VP2 and VP3 share a common C terminus and stop codon, but begin with a different start codon. The N termini of VP1 and VP2 play important roles in infection and contain motifs that are highly homologous to a phospholipase A2 (PLA2) domain and nuclear localization signals (NLSs). These elements are conserved in almost all parvoviruses. (Johnson et al., 2010a).
Whereas VP1 is translated from the minor spliced mRNA, while VP2 and VP3 are translated from the major spliced mRNA. The minor spliced product is approximately 10-fold less abundant than the major spliced mRNA. Thus, there is much less VP1 than VP2 and VP3 resulting in a capsid stoichiometric ratio of 1:1:10. The N terminus of VP1 has an extension of 65 amino acids including an additional extension of 138 N-terminal amino acids forming the unique portion of VP1. It contains a motif of about 70 amino acids that is highly homologous to phospholipase A2 (PLA2) domain. Furthermore, there are nuclear localization sequences (BR)(+), which are supposed to be necessary for endosomal escape and nuclear entry. (Bleker, Pawlita, & Kleinschmidt, 2006), (DiPrimio, Asokan, Govindasamy, Agbandje-McKenna, & Samulski, 2008), (Johnson et al., 2010a)
VP1up
VP1up protein is derived from the unique N-terminal region of VP1 protein. It contains a Phospholipase A2 motif which is essential for successful infection [Canaan et al., 2004; Zadori et al., 2001; Girod et al., 2002]
The Freiburg iGEM Team 2010 created this part in order integrate motifs, which are desired to be surface exposed, into the VP1 open reading frame. For this purpose VP1up needs to be fused to the N-terminus of the motif of interest, followed by coupling the resulting construct to VP2/3 (BBa_K404150, [AAV2]-VP23). For better infectivity a nuclear localization signal (Part:BBa_K404153, [AAV2]-NLS) (Grieger et al., 2007).
NLS
NLS are located in basic regions on the N terminus of VP2 (35 aa) and VP1 (172 aa) and mediate genome delivery into the nucleus and transduction [Hoque et al.,1999; Grieger et al., 2006]. Nuclear localisation sequence is hydrophilic and contains ß-turn and coil regions [Kalderon, et al, 1984]. It was also described in CPV and MVM viruses. Compared to CPV, MVM virus contains several NLS within the capsid, which are activated at different infection stages [Lombardo et al., 2000; Lombardo et al., 2002]
BAP
The Biotinylation Acceptor Peptide (BAP) is a 15 amino acid long peptide identified by Schatz J., 1993 in an library screening approach. This peptide with the sequence 5' - GLNDIFEAQKIEWHE - 3' contains a central lysine that is specifically biotinylated by the prokaryotic enzyme biotin holenzyme synthetase, encoded in the BirA gene of E. coli. Specific biotinylation of this peptide sequence can be performed in vivo by contransfecting a plasmid with the BirA gene as described for the AAV in Arnold et al.; 2006 or by an in vitro coupling approach using the purified Escherichia coli enzyme biotin ligase (BirA).

Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 3149
Illegal XhoI site found at 698
Illegal XhoI site found at 884 - 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 665
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI site found at 3735
Illegal SapI site found at 2586