Difference between revisions of "Part:BBa K404222"
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
<partinfo>BBa_K404222 short</partinfo> | <partinfo>BBa_K404222 short</partinfo> | ||
| + | {| style="color:black" cellpadding="6" cellspacing="1" border="2" align="left" | ||
| + | ! colspan="2" style="background:#66bbff;"|[https://parts.igem.org/Part:BBa_K404222 (AAV2)-Rep-VP123_p5-TATAless (ViralBrick-453-His-Tag)] | ||
| + | |- | ||
| + | |'''BioBrick Nr.''' | ||
| + | |[https://parts.igem.org/Part:BBa_K404222 BBa_K404222] | ||
| + | |- | ||
| + | |'''RFC standard''' | ||
| + | |[https://parts.igem.org/Help:Assembly_standard_25 RFC 25] | ||
| + | |- | ||
| + | |'''Requirement''' | ||
| + | |pSB1C3<br> | ||
| + | |- | ||
| + | |'''Source''' | ||
| + | | | ||
| + | |- | ||
| + | |'''Submitted by''' | ||
| + | |[http://2010.igem.org/Team:Freiburg_Bioware FreiGEM 2010] | ||
| + | |} | ||
| + | <br/><br/><br/><br/><br/><br/><br/><br/><br/><br/><br/><br/><br/> | ||
[[Image:Freiburg10_Rep-VP123_P5-TATA-less 453-His.png|thumb|center|480px]]<br> | [[Image:Freiburg10_Rep-VP123_P5-TATA-less 453-His.png|thumb|center|480px]]<br> | ||
Latest revision as of 02:48, 28 October 2010
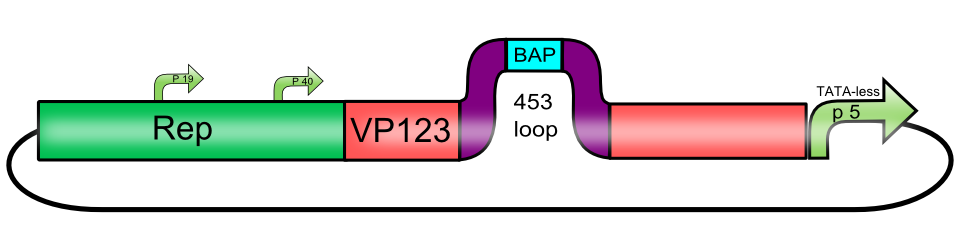
[AAV2]-Rep-VP123_P5-TATAless (ViralBrick-453-His-Tag)
| (AAV2)-Rep-VP123_p5-TATAless (ViralBrick-453-His-Tag) | |
|---|---|
| BioBrick Nr. | BBa_K404222 |
| RFC standard | RFC 25 |
| Requirement | pSB1C3 |
| Source | |
| Submitted by | [http://2010.igem.org/Team:Freiburg_Bioware FreiGEM 2010] |
Usage and Biology
Protein tagging via Histidine Tags is a widely used method for protein purification: Multiple histidine residues (most commonly: Six) are being fused tot he end of the targeting protein.
The high binding affinity of Histidine towards metal is being exploited for the purification of proteins via the so called „Immobilized Metal Ion Affinity Chromatography“ (IMAC): Multiple histidine residues (most commonly: Six) are being fused to the end of the targeting protein. A cell extract containing the recombinant protein ist then applied to a collumn containing immobilized Ni2+-Ions. The His-tags covalently bind the Ni-Ions while other cellular proteins can be washed oft he collumn. The purified proteins can then be eluted with Imidazol, which displaces the histidine residues.(Smith et al. 1988), (Hoffmann & Roeder 1991)
Since the aim behind engineering therapeutic AAV vectors is a safe administration to human patients, it is important to consider a convenient way of purifying the virus particles. Contamination by cellular proteins could cause toxic side effects or a strong immune response. Koerber et al. have first inserted a His-tag into a surface-exposed loop at amino acid position 587 in the Cap protein and successfully purified recombinant virsuses using IMAC (Koerber et al. 2007). For our Virus Construction Kit, we provide the His-tag motif in the ViralBrick standard, allowing for an easy insertion into the 453 and/or 587 loop. If the modified capsid bearing a His-tag is being cotransfected with a wild type capsid for the production of mosaic viruses, IMAC helps to not only purify the produced viral particles but also to enrich particles which actually contain the modified proteins.

Capsid
The AAV capsid consists of 60 capsid protein subunits. The three cap proteins VP1, VP2, and VP3 are encoded in an overlapping reading frame. Arranged in a stoichiometric ratio of 1:1:10, they form an icosahedral symmetry. The mRNA encoding for the cap proteins is transcribed from p40 and alternative spliced to minor and major products. Alternative splicing and translation initiation of VP2 at a nonconventional ACG initiation codon promote the expression of VP1, VP2 and VP3. The VP proteins share a common C terminus and stop codon, but begin with a different start codon. The N termini of VP1 and VP2 play important roles in infection and contain motifs that are highly homologous to the phospholipase A2 (PLA2) domain and nuclear localization signals (BR)(+).References
DiPrimio, Asokan, Govindasamy, Agbandje-McKenna, & Samulski, June 2008. Surface loop dynamics in adeno-associated virus capsid assembly. Journal of virology, 167(1), 5178–5189
Figure 1: The VP proteins are encoded in an overlapping open reading frame. Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 3635
Illegal XhoI site found at 1913
Illegal XhoI site found at 2099 - 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI site found at 4161
Illegal BsaI site found at 4343
Illegal BsaI site found at 4380
Illegal SapI site found at 3048