Difference between revisions of "Part:BBa K404303"
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
<partinfo>BBa_K404303 short</partinfo> | <partinfo>BBa_K404303 short</partinfo> | ||
| + | <br/><br><br> | ||
| + | {| style="color:black" cellpadding="6" cellspacing="1" border="2" align="left" | ||
| + | ! colspan="2" style="background:#66bbff;"|[https://parts.igem.org/Part:BBa_K404303 [Z-EGFR-1907_Short-Linker ] | ||
| + | |- | ||
| + | ! colspan="2"|[[Image:Freiburg10_Vectorplasmid composite 5.png|300px]] | ||
| + | |- | ||
| + | |'''BioBrick Nr.''' | ||
| + | |[https://parts.igem.org/Part:BBa_K404303 BBa_K404303] | ||
| + | |- | ||
| + | |'''RFC standard''' | ||
| + | |[https://parts.igem.org/Help:Assembly_standard_25 RFC 25] | ||
| + | |- | ||
| + | |'''Requirement''' | ||
| + | |pSB1C3<br> | ||
| + | |- | ||
| + | |'''Source''' | ||
| + | |pAAV_MCS: provided by Stratagene<br/> | ||
| + | [https://parts.igem.org/Part:BBa_K243004 BBa_K243004] | ||
| + | |- | ||
| + | |'''Submitted by''' | ||
| + | |[http://2010.igem.org/Team:Freiburg_Bioware FreiGEM 2010] | ||
| + | |} | ||
| + | <br><br><br><br><br><br><br><br><br><br><br><br><br><br><br> | ||
<html> | <html> | ||
| Line 28: | Line 51: | ||
margin-left:0cm; | margin-left:0cm; | ||
font-size:13.5pt; | font-size:13.5pt; | ||
| − | font-family:" | + | font-family:"Times New Roman","serif"; |
font-weight:bold;} | font-weight:bold;} | ||
a:link, span.MsoHyperlink | a:link, span.MsoHyperlink | ||
| Line 40: | Line 63: | ||
margin-left:0cm; | margin-left:0cm; | ||
font-size:12.0pt; | font-size:12.0pt; | ||
| − | font-family:" | + | font-family:"Times New Roman","serif";} |
p.MsoListParagraph, li.MsoListParagraph, div.MsoListParagraph | p.MsoListParagraph, li.MsoListParagraph, div.MsoListParagraph | ||
{margin-top:0cm; | {margin-top:0cm; | ||
| Line 78: | Line 101: | ||
{mso-style-name:"Heading 3 Char"; | {mso-style-name:"Heading 3 Char"; | ||
mso-style-link:"Heading 3"; | mso-style-link:"Heading 3"; | ||
| − | font-family:" | + | font-family:"Times New Roman","serif"; |
font-weight:bold;} | font-weight:bold;} | ||
span.apple-converted-space | span.apple-converted-space | ||
| Line 103: | Line 126: | ||
<p class="MsoNormal" | <p class="MsoNormal" | ||
style="margin: 4.8pt 0cm 6pt; line-height: 18pt;"><b><span | style="margin: 4.8pt 0cm 6pt; line-height: 18pt;"><b><span | ||
| − | style="font-size: 10pt; font-family: "Arial","sans-serif"; color: black;">This | + | style="font-size: 10pt; font-family: "Arial","sans-serif"; color: black;"><br> |
| − | composite part contains | + | This composite part contains |
| − | affibody and short linker.</span>< | + | affibody and short linker.</span><br> |
| − | < | + | <small><small><span |
| − | + | style="font-size: 14.5pt; font-family: "Arial","sans-serif"; color: black;"><small><small><br> | |
| − | style="font-size: 14.5pt; font-family: "Arial","sans-serif"; color: black;"> | + | Short Linker (Gl</small><small>y-Gly-Ser-Gly)</small></small></span></small></small></b><span |
| − | < | + | style="font-size: 10pt; font-family: "Arial","sans-serif"; color: black;"><br> |
| − | + | In general, linkers can be used to avoid interaction | |
| − | + | ||
| − | + | ||
| − | < | + | |
| − | + | ||
| − | style="font-size: 10pt; font-family: "Arial","sans-serif"; color: black;"> | + | |
| − | general, linkers can be used to avoid interaction | + | |
between different proteins to connect them or add additional space | between different proteins to connect them or add additional space | ||
between | between | ||
| Line 128: | Line 145: | ||
</body> | </body> | ||
</html> | </html> | ||
| + | |||
Latest revision as of 21:11, 27 October 2010
Z-EGFR-1907_Short-Linker
| [Z-EGFR-1907_Short-Linker | |
|---|---|
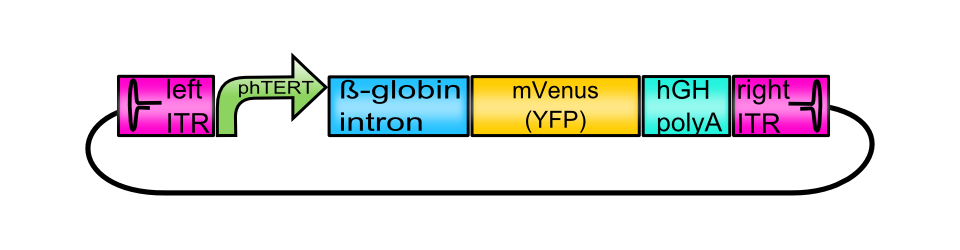
| |
| BioBrick Nr. | BBa_K404303 |
| RFC standard | RFC 25 |
| Requirement | pSB1C3 |
| Source | pAAV_MCS: provided by Stratagene |
| Submitted by | [http://2010.igem.org/Team:Freiburg_Bioware FreiGEM 2010] |
This composite part contains
affibody and short linker.
Short Linker (Gly-Gly-Ser-Gly)
In general, linkers can be used to avoid interaction
between different proteins to connect them or add additional space
between
them. In this content it is important that the linker itself has no
influence
on the connected proteins and has the right length to avoid sterical
interference. The sequence of the short linker encodes the aminoacids
Gly-Gly-Ser-Gly.
Affibody ZEGFR:1907
Affibodies are small (6 kDa), soluble high-affinity proteins. They are derived from the IgG-binding B domain of the Staphylococcal protein A, which was engineered to specifically bind to certain peptides or proteins. This so-called Z domain consists of an antiparallel three-helix bundle and is advantageous due to its proteolytic and thermodynamic stability, its good folding properties and the ease of production via recombinant bacteria (Nord et al. 1997). Affibodies can be used for example for tumor targeting (Wikman et al. 2004) and diagnostic imaging applications(Friedman et al. 2008)(Orlova et al. 2007). The ZEGFR:1907 Affibody was engineered to specifically bind the EGF receptor with an affinity determined to be KD = 2.8 nM (Friedman et al. 2008).
The EGF receptor is overexpressed in certain types of tumors, e.g. in breast (Walker and Dearing 1999), lung (Hirsch et al. 2003) and bladder (Colquhoun and Mellon 2002) carcinomas, and is therefore a suitable target for cancer imaging or therapeutic applications. Because of their good tumor uptake, and their property to become internalized into the target cells with an efficiency of 19 – 24% within one hour – compared to 45% of the natural ligand EGF - the ZEGFR:1907 Affibody was chosen for therapeutic applications by the Freiburg iGEM Team 2010 (Göstring et al. 2010; Friedman et al. 2008).
|
Figure 29: Nucelotide sequence of designed ZEGFR:1907 |
References
Colquhoun, a J, and J K Mellon. 2002. Epidermal growth factor receptor and bladder cancer. Postgraduate medical journal 78, no. 924 (October): 584-9. doi:10.1136/pmj.78.924.584. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1742539&tool=pmcentrez&rendertype=abstract.
Friedman, Mikaela, Anna Orlova, Eva Johansson, Tove L J Eriksson, Ingmarie Höidén-Guthenberg, Vladimir Tolmachev, Fredrik Y Nilsson, and Stefan Ståhl. 2008. Directed evolution to low nanomolar affinity of a tumor-targeting epidermal growth factor receptor-binding affibody molecule. Journal of molecular biology 376, no. 5: 1388-402. doi:10.1016/j.jmb.2007.12.060. http://www.ncbi.nlm.nih.gov/pubmed/18207161.
Göstring, Lovisa, Ming Tsuey Chew, Anna Orlova, Ingmarie Höidén-guthenberg, Anders Wennborg, Jörgen Carlsson, and Fredrik Y Frejd. 2010. Quantification of internalization of EGFR-binding Affibody molecules: Methodological aspects. International Journal of Oncology 36, no. 4 (March): 757-763. doi:10.3892/ijo_00000551. http://www.spandidos-publications.com/ijo/36/4/757.
Hirsch, Fred R, Marileila Varella-Garcia, Paul a Bunn, Michael V Di Maria, Robert Veve, Roy M Bremmes, Anna E Barón, Chan Zeng, and Wilbur a Franklin. 2003. Epidermal growth factor receptor in non-small-cell lung carcinomas: correlation between gene copy number and protein expression and impact on prognosis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 21, no. 20 (October): 3798-807. doi:10.1200/JCO.2003.11.069. http://www.ncbi.nlm.nih.gov/pubmed/12953099.
Nord, K, E Gunneriusson, J Ringdahl, S Ståhl, M Uhlén, and P A Nygren. 1997. Binding proteins selected from combinatorial libraries of an alpha-helical bacterial receptor domain. Nature biotechnology 15, no. 8 (August): 772-7. doi:10.1038/nbt0897-772. http://www.ncbi.nlm.nih.gov/pubmed/9255793.
Orlova, Anna, Vladimir Tolmachev, Rikard Pehrson, Malin Lindborg, Thuy Tran, Mattias Sandström, Fredrik Y Nilsson, Anders Wennborg, Lars Abrahmsén, and Joachim Feldwisch. 2007. Synthetic affibody molecules: a novel class of affinity ligands for molecular imaging of HER2-expressing malignant tumors. Cancer research 67, no. 5 (March): 2178-86. doi:10.1158/0008-5472.CAN-06-2887. http://www.ncbi.nlm.nih.gov/pubmed/17332348.
Walker, R a, and S J Dearing. 1999. Expression of epidermal growth factor receptor mRNA and protein in primary breast carcinomas. Breast cancer research and treatment 53, no. 2 (January): 167-76. http://www.ncbi.nlm.nih.gov/pubmed/10326794.
Wikman, M, a-C Steffen, E Gunneriusson, V Tolmachev, G P Adams, J Carlsson, and S Ståhl. 2004. Selection and characterization of HER2/neu-binding affibody ligands. Protein engineering, design & selection : PEDS 17, no. 5 (May): 455-62. doi:10.1093/protein/gzh053. http://www.ncbi.nlm.nih.gov/pubmed/15208403.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]

