Difference between revisions of "Part:BBa K404212"
| Line 6: | Line 6: | ||
===Usage and Biology=== | ===Usage and Biology=== | ||
| + | <div style="width:965px; height:400px; "> | ||
| + | <div style="float:left; width:460px; height:auto; margin: 0px 5px 0px 5px; text-align:justify;"> | ||
| + | |||
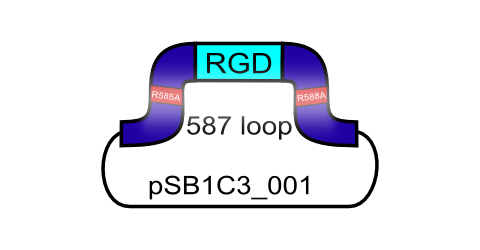
Integrins are transmembrane proteins that, among other functions, mediate cell attachment to surrounding tissues. They bind to a motif consisting of the amino acids arginine, glycine and aspartic acid (RGD in one-letter code). Because Integrin is highly expressed in many tumor cell lines (Albelda et al., 1990), (Damjanovich, Albelda, Mette, & Buck, 1992), (Lessey et al., 1995), (Smythe, LeBel, Bavaria, Kaiser, & Albelda, 1995), (Gladson & Cheresh, 1991), AAV particles displaying the RGD motif on various positions in their capsid proteins have been created by (Shi et al., 2003). Particles displaying RGD at amino acid positions 584 & 588 as well as 453 or 587 (Boucas et al., 2009) showed transduction efficiencies similar to wt AAV, even when the cells’ HSPG receptors were blocked by heparin sulfate or when the natural HSPG binding motif on the capsid surface was knocked out. To further broaden the area of therapeutic application, we created a ViralBrick containing the RGD motive to specifically target cells with low HSPG-/high Integrin expression.<br> | Integrins are transmembrane proteins that, among other functions, mediate cell attachment to surrounding tissues. They bind to a motif consisting of the amino acids arginine, glycine and aspartic acid (RGD in one-letter code). Because Integrin is highly expressed in many tumor cell lines (Albelda et al., 1990), (Damjanovich, Albelda, Mette, & Buck, 1992), (Lessey et al., 1995), (Smythe, LeBel, Bavaria, Kaiser, & Albelda, 1995), (Gladson & Cheresh, 1991), AAV particles displaying the RGD motif on various positions in their capsid proteins have been created by (Shi et al., 2003). Particles displaying RGD at amino acid positions 584 & 588 as well as 453 or 587 (Boucas et al., 2009) showed transduction efficiencies similar to wt AAV, even when the cells’ HSPG receptors were blocked by heparin sulfate or when the natural HSPG binding motif on the capsid surface was knocked out. To further broaden the area of therapeutic application, we created a ViralBrick containing the RGD motive to specifically target cells with low HSPG-/high Integrin expression.<br> | ||
| − | + | </div> | |
| + | |||
| + | <html><div style="float:right; width:480px; height:auto; "><img src="https://static.igem.org/mediawiki/parts/5/59/Freiburg10_ViralBrick_motif_RGD.png" width="460" | ||
| + | height="auto"/></div></div><br><br><br><br></html> | ||
| + | |||
<table border="1" style="float:right; width:480px; height:auto;> | <table border="1" style="float:right; width:480px; height:auto;> | ||
Revision as of 17:22, 26 October 2010
ViralBrick-587KO-RGD
The RGD integrin binding motif including a knockout of the natural HSPG tropism, ready for insertion into the 587 loop
Usage and Biology
Integrins are transmembrane proteins that, among other functions, mediate cell attachment to surrounding tissues. They bind to a motif consisting of the amino acids arginine, glycine and aspartic acid (RGD in one-letter code). Because Integrin is highly expressed in many tumor cell lines (Albelda et al., 1990), (Damjanovich, Albelda, Mette, & Buck, 1992), (Lessey et al., 1995), (Smythe, LeBel, Bavaria, Kaiser, & Albelda, 1995), (Gladson & Cheresh, 1991), AAV particles displaying the RGD motif on various positions in their capsid proteins have been created by (Shi et al., 2003). Particles displaying RGD at amino acid positions 584 & 588 as well as 453 or 587 (Boucas et al., 2009) showed transduction efficiencies similar to wt AAV, even when the cells’ HSPG receptors were blocked by heparin sulfate or when the natural HSPG binding motif on the capsid surface was knocked out. To further broaden the area of therapeutic application, we created a ViralBrick containing the RGD motive to specifically target cells with low HSPG-/high Integrin expression.

Results

Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 10
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]