Difference between revisions of "Part:BBa K346003:Experience"
JjunyiJiao (Talk | contribs) (→Applications of BBa_K346003) |
|||
| Line 1: | Line 1: | ||
| − | |||
__NOTOC__ | __NOTOC__ | ||
This experience page is provided so that any user may enter their experience using this part.<BR>Please enter | This experience page is provided so that any user may enter their experience using this part.<BR>Please enter | ||
how you used this part and how it worked out. | how you used this part and how it worked out. | ||
| − | === | + | ===construction of BBa_K346003=== |
| + | |||
| + | [[Image:mbp4.jpg]] | ||
| + | |||
| + | |||
| + | To be specific, the entire coding region of the MBP for standard part was amplified by PCR from full length MerR with two pairs of primers. Two of these primers encoded a three-residue bridge, SSG, which does not occur in MerR and was added to afford some flexibility in the loop connecting the two dimerization helix. The two PCR products were digested with EcoR I / BamH I, or BamH I / Pst I and cloned into EcoR I / Pst I -digested pSB1K3 in one step (fig), which was verified by DNA sequencing.Then the RBS and terminator is added. | ||
| + | |||
| + | Based on the same strategy, MBP-His6 was constructed by using two different pairs of primers, which is used for MBP expression test by western blot. The two PCR products were digested with Nde I / BamH I, or BamH I / Xho I and cloned into Nde I / Xho I -digested pET 21a, which contains a region encoding six histines, in one step to construct pET 21a – mbp , which was verified by DNA sequencing. | ||
| + | |||
| + | |||
| + | ==Expression Experiment and Function Test: == | ||
| + | |||
| + | To test the function of this part, both expression experiment and function test is necessary. We have verified the size of the expressed proteins with SDS-page and Western blot. Besides, to test the efficiency of mercury binding, we also carried out the function test with ICP-AES with the mercury gradient from 10^-8M to 10^-6M. | ||
| + | |||
| + | |||
| + | == Results: == | ||
| + | |||
| + | '''Expression of proteins''' | ||
| + | |||
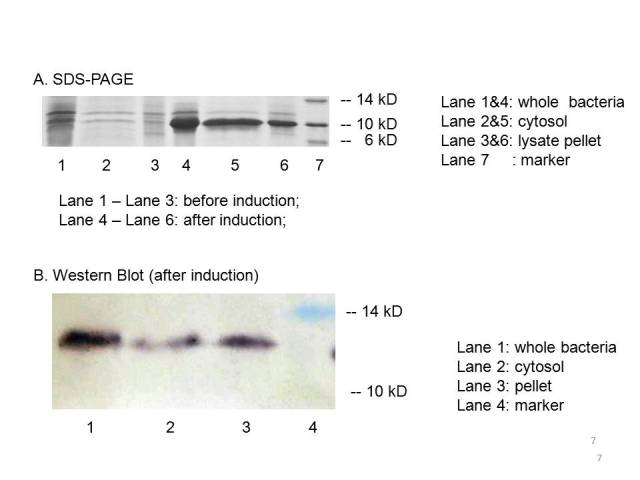
| + | The plasmid PET21a-mbp is transferred to E.coli strain BL21, which can generate T7polyerase when induced with IPTG. Both induced cells and uninduced cells(as control) are centrifuged to get the cytosol, the periplasm and the membrane separated. The SDS-page and Western blot of the expressed proteins show that induced cells expressed an identical IPTG-inducible protein at the proper place with the size of ~12kD which consists with the predicted size, indicating that the engineered MBP can be expressed in the cytosol. | ||
| + | |||
| + | [[Image:result of MBP.jpg]] | ||
| + | |||
| + | '''Function test''' | ||
| + | |||
| + | Having made sure that the protein can express normally in the cytosol, the function tests experiment are carried out with ICP-AES. To test the efficiency of mercury absorption of MBP in different concentration of mercury, the concentration gradient is set from 10^-8M to 10^-6M, the results are shown in figure 3. It is obvious that the efficiency of MBP increases with the increase of the mercury concentration and it can binding Hg(II) with high efficiency and high sensitivity from the concentration of 10^-7 M compared to that of the control. | ||
| + | |||
| + | [[Image:FIGURE 3.jpg]] | ||
===User Reviews=== | ===User Reviews=== | ||
Revision as of 00:24, 25 October 2010
This experience page is provided so that any user may enter their experience using this part.
Please enter
how you used this part and how it worked out.
construction of BBa_K346003
To be specific, the entire coding region of the MBP for standard part was amplified by PCR from full length MerR with two pairs of primers. Two of these primers encoded a three-residue bridge, SSG, which does not occur in MerR and was added to afford some flexibility in the loop connecting the two dimerization helix. The two PCR products were digested with EcoR I / BamH I, or BamH I / Pst I and cloned into EcoR I / Pst I -digested pSB1K3 in one step (fig), which was verified by DNA sequencing.Then the RBS and terminator is added.
Based on the same strategy, MBP-His6 was constructed by using two different pairs of primers, which is used for MBP expression test by western blot. The two PCR products were digested with Nde I / BamH I, or BamH I / Xho I and cloned into Nde I / Xho I -digested pET 21a, which contains a region encoding six histines, in one step to construct pET 21a – mbp , which was verified by DNA sequencing.
Expression Experiment and Function Test:
To test the function of this part, both expression experiment and function test is necessary. We have verified the size of the expressed proteins with SDS-page and Western blot. Besides, to test the efficiency of mercury binding, we also carried out the function test with ICP-AES with the mercury gradient from 10^-8M to 10^-6M.
Results:
Expression of proteins
The plasmid PET21a-mbp is transferred to E.coli strain BL21, which can generate T7polyerase when induced with IPTG. Both induced cells and uninduced cells(as control) are centrifuged to get the cytosol, the periplasm and the membrane separated. The SDS-page and Western blot of the expressed proteins show that induced cells expressed an identical IPTG-inducible protein at the proper place with the size of ~12kD which consists with the predicted size, indicating that the engineered MBP can be expressed in the cytosol.
Function test
Having made sure that the protein can express normally in the cytosol, the function tests experiment are carried out with ICP-AES. To test the efficiency of mercury absorption of MBP in different concentration of mercury, the concentration gradient is set from 10^-8M to 10^-6M, the results are shown in figure 3. It is obvious that the efficiency of MBP increases with the increase of the mercury concentration and it can binding Hg(II) with high efficiency and high sensitivity from the concentration of 10^-7 M compared to that of the control.
User Reviews
UNIQ0466e51ff1ecca4d-partinfo-00000000-QINU UNIQ0466e51ff1ecca4d-partinfo-00000001-QINU