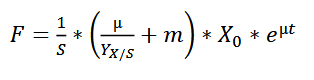Difference between revisions of "Part:BBa K3264000"
(→Production of NC-2Rep in Supernatant) |
Jocomodaro (Talk | contribs) |
||
| Line 238: | Line 238: | ||
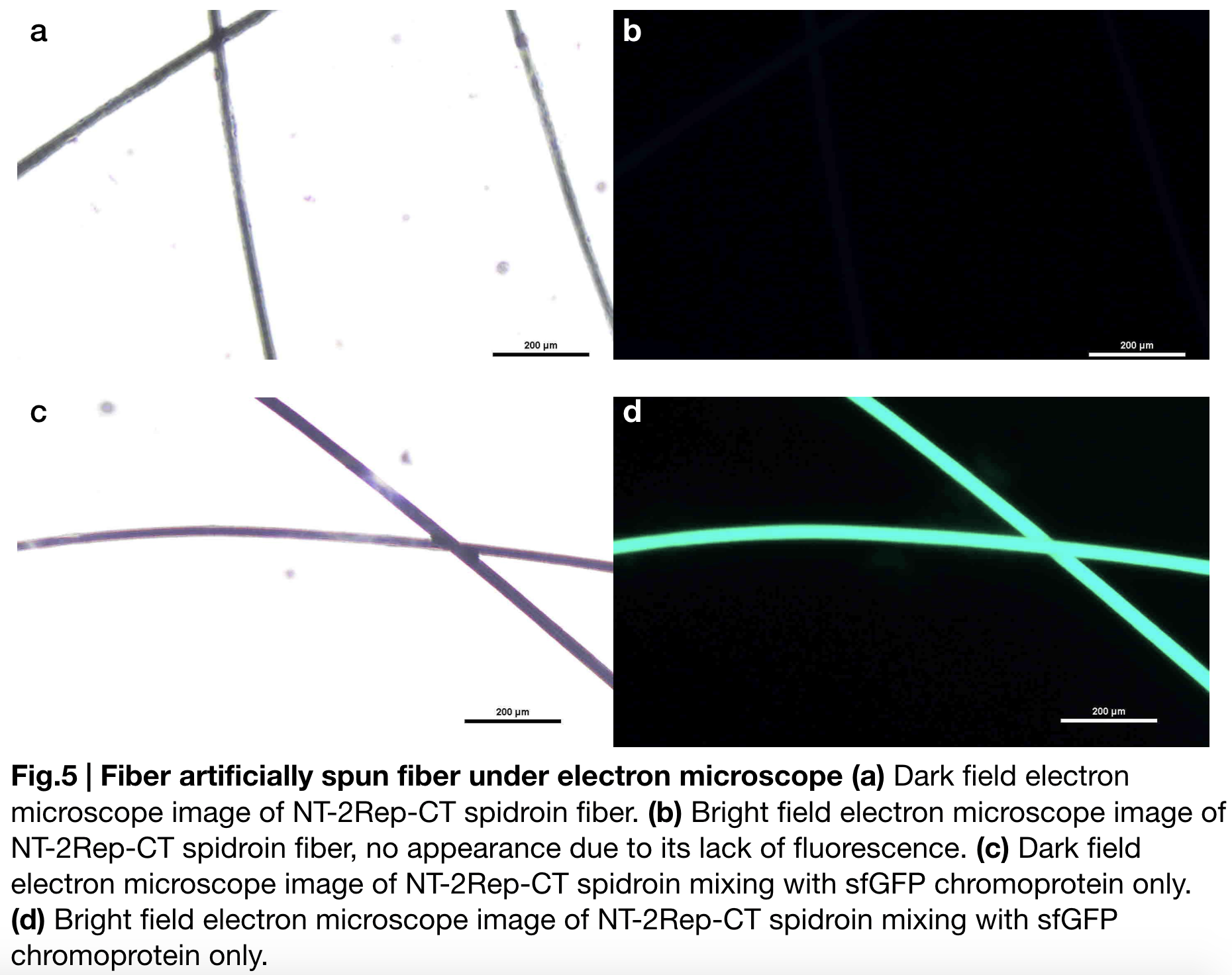
Aiming to verify the distribution uniformity of NT2RepCT after spinning into fiber, we looked at our artificial spun silks by NT-2Rep-CT spidroin and concocting NT-2Rep-CT spidroin with sfGFP chromoprotein under electron microscope '''(Fig.5)''', the role of sfGFP chromoprotein here is for us to clearly observe our silk under electron microscope. We randomly selected the cross section in our continuous silk, it is clearly shown that the fiber circularity is qualified as a uniformly distributed silk. Without sfGFP mixed, the spider silk spun by NT-2Rep-CT spidroin only can not be observed from the positive electron microscope image due to its lack of fluorescence. | Aiming to verify the distribution uniformity of NT2RepCT after spinning into fiber, we looked at our artificial spun silks by NT-2Rep-CT spidroin and concocting NT-2Rep-CT spidroin with sfGFP chromoprotein under electron microscope '''(Fig.5)''', the role of sfGFP chromoprotein here is for us to clearly observe our silk under electron microscope. We randomly selected the cross section in our continuous silk, it is clearly shown that the fiber circularity is qualified as a uniformly distributed silk. Without sfGFP mixed, the spider silk spun by NT-2Rep-CT spidroin only can not be observed from the positive electron microscope image due to its lack of fluorescence. | ||
| − | + | ===Team CNPEM-Brazil 2024 literature review=== | |
| + | Our objective is to deliver a comprehensive and updated literature review about the Nt2RepCt, which holds substantial biotechnological potential for diverse applications across multiple fields. | ||
| + | *'''Coacervate Microdroplets:''' A study published in June 2023 focuses on the construction of coacervate microdroplets using recombinant spidroin NT2RepCT. This research emphasizes the ability of these microdroplets to respond to environmental stimuli, making them suitable for simulating advanced life behaviors. The morphology of these droplets can be manipulated by altering conditions such as protein concentration, pH, and temperature, which is crucial for understanding material properties and phase behavior in artificial cell systems. [https://pubs.rsc.org/en/content/articlelanding/2023/tb/d3tb00878a] | ||
| + | *'''Nt2RepCt hydrogel:''' This study from May, 2023 discusses the innovative development of hydrogels derived from recombinant spider silk proteins, which can be tailored for specific biomedical applications. The researchers demonstrate how these hydrogels can be engineered to possess adjustable mechanical and physical properties, making them suitable for controlled drug release and 3D cell culture environments. The study emphasizes the potential of these spider silk-based hydrogels to mimic the extracellular matrix, thereby enhancing cell growth and interaction. This work highlights the versatility and biocompatibility of spider silk proteins, positioning them as promising materials for future applications in tissue engineering and regenerative medicine. [https://doi.org/10.1002/adfm.202303622] | ||
| + | *'''Synthetic spider silk:''' A study published in July, 2024 addresses the challenge of replicating the exceptional mechanical properties of natural spider silk through synthetic methods. It introduces a novel approach using multiarm polyethylene glycol (PEG) to create synthetic spider silk that retains biomimetic qualities while enhancing tensile strength and extensibility. The study highlights the versatility of bioconjugation techniques in modifying protein structures, facilitating spidroin crosslinking, and chemical functionalization. By employing this method, the researchers aim to improve the toughness of the fibers without needing to develop new mini-spidroin constructs, thus potentially mitigating the adverse effects of environmental conditions on synthetic spider silk applications. [https://doi.org/10.1002/adfm.202409487] | ||
| + | *'''Studying structural variants''': The study from september, 2023, investigates the role of a spider silk protein, NT2RepCT, in promoting liquid-liquid phase separation, a critical process in the formation of intracellular condensates within yeast cells (''Saccharomyces cerevisiae''). The study explores how different structural variants of NT2RepCT behave under varying intracellular conditions, revealing that factors such as molecular crowding and pH significantly influence the condensation properties and growth of these protein assemblies. By conducting parallel in vivo and in vitro experiments, the researchers demonstrate that yeast cells can provide a unique environment for studying protein condensation mechanisms, which could inform future designs of biomolecular materials for synthetic biology applications. [https://pubs.acs.org/doi/full/10.1021/acssynbio.3c00374] | ||
<partinfo>BBa_K3264000 parameters</partinfo> | <partinfo>BBa_K3264000 parameters</partinfo> | ||
<!-- --> | <!-- --> | ||
Revision as of 23:19, 23 September 2024
NT-2Rep-CT
NT-2Rep-CT is the recombinant spider silk protein (spidroin) that can spin into artificial spider silk fiber. This part is in the part collection where we provide improved recombinant chromoproteins and spidroins based on the functions of different regions of naturally occurring spidroin. By carrying out different mixing of the proteins of different combinations, we can produce artificially spun silk of different colors and properties.
The part collection includes: Parts that are different kinds of spidroins for silk spining. BBa_K3264000 BBa_K3264001 BBa_K3264002 BBa_K3264003 BBa_K3264004 BBa_K3264005 BBa_K3264007 BBa_K3264009 BBa_K3264018. BBa_K3264019. Parts that for combining with the spidroins to form color silks BBa_K3264012. BBa_K3264013. BBa_K3264014. BBa_K3264015. BBa_K3264016. BBa_K3264017. Part for adding more extensive repetitive region to the spidroin protein BBa_K3264011.
Our part collection can help and inspire future teams to further design recombinant spider silks of more variety and give silks diversified color and property to fulfill the collection.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
iGEM 2023 SHSBNU - Contribution
Team: SHSBNU-China
Production of NC-2Rep in Supernatant
iGEM 2019 team GreatBay_SZ designed a project to produce recombinant spidroins (silk proteins) from E.coli and spin them into silk. Our team SHSBNU-China this year concentrated on using microbial as a biological chasis to yield polymers, which has some similarity with the previous GreatBay_SZ project. According to this, we synthesized the sequence uploaded and constructed it onto pET28a(+) plasmid to repeat the experiments at first.
The new plasmid was named as pET28a(+)-NT-2Rep-CT, which was transfected into E.coli BL21(DE3) for efficient protein expression. However, we noticed two essential problems for GreatBay_SZ in their experiment: 1) They elucidated to suggest a complex growth medium that integrate a specific trace metal solution to cultivate the bacteria. 2) They found the spidroin continually altered from soluble to insoluble state. Our team SHSBNU_China discussed about the two problems, we thought that although the bacteria can grow in the given condition, but it could bring great inconvenience for the subsequent experiment. Especially when the silk is indeed used in the textile industry, is it safe for human beings and our environment? Besides, based on our experience in titin program this year, we believe the insoluble state can be improved by changing the expression condition.
According to the above discussion, we tried to cultivate the bacteria in standard LB culture, and we set the following conditions: 1) induction for 20 hours under 16℃ 2) induction for 4 hours under 37℃
As we can see in the SDS-PAGE, spidroin can be expressed using standard LB culture, and although it precipitated in the inclusion body under 37℃, it became soluble after induction for 20 hours under 16℃. Based on these results, we believe we offered a new solution for the production of spidroin, which solves the problem of specific culture conditions and solubility.

iGEM 2022 UCopenhagen - Contribution
Designers: Akila Ravikumar and Matteo Soana
Team: Netlantis
Large scale production of NT-2rep-CT
As part of our project, we produced two minispidroins whose production was scaled up. One of these minispidroins had the same amino acid sequence as this part (we used the same scale up strategy for all of them). Herein, we provide information regarding the fermentation of this protein using E.coli in a fed-batch cultivation. Our entire fermentation process was based on the methods of the paper: “High-yield production of a super-soluble miniature spidroin for biomimetic high-performance materials” by Schmuck et al., 2021.
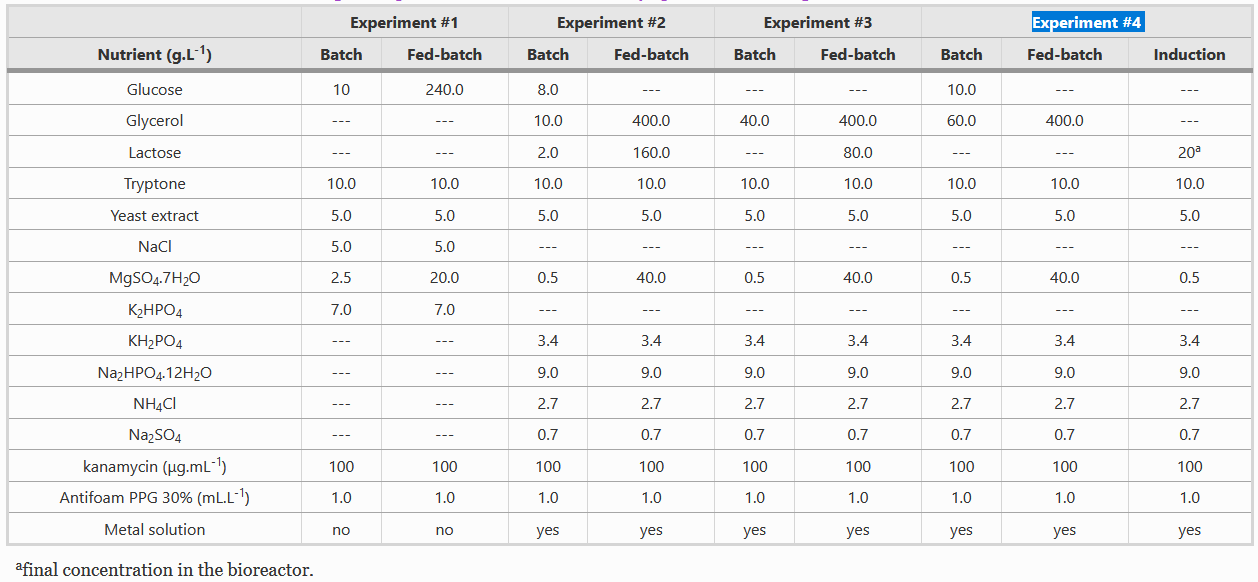
Composition of Batch and Fed-batch media
Instead of LB media, we used a complex growth medium that integrated a specific trace metal solution. The authors obtained this media from experiment #4 (see below) of another paper: A.J. da Silva, A.C.L. Horta, A.M. Velez, M.R.C. Iemma, C.R. Sargo, R.L.C. Giordano, M.T.M. Novo, R.C. Giordano, T.C. Zangirolami, Springerplus 2 (2013) 1–12.
For our bioreactor, we always started cultures with 1 L of batch media and set the system such that 500 mL of fed-batch media would enter the system at a specific rate and we used Silica antifoam.
The feeding rate should follow the formula:
wherein,
F - the rate of feeding (l h-1 )
S - concentration of the glycerol in the feed (g l-1 )
µ - specific growth rate (h-1)
YX/S - biomass yield on the substrate (g g-1 )
m - specific maintenance coefficient (g g-1 h -1 )
X - biomass concentration (g l-1 )
For the fermentation of this minispidroin protein, the authors set µ to 0.1, m to 0.025, and YX/S to 0.622.
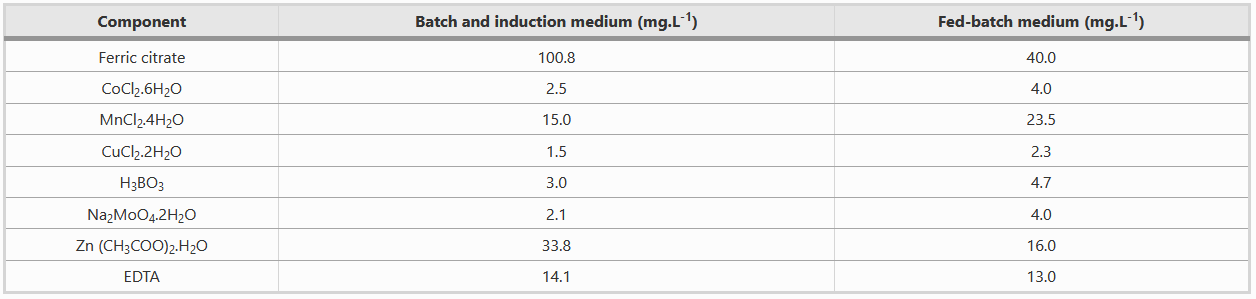
Composition of Metal solution
Both batch and fed-batch media have to be complemented with two different metal solutions, as shown in the table below.
Note: Be careful with the preparation of ferric citrate because residual non solubilised Fe(3+), being rust, can be quite problematic for the small fermentor tubings and clog them, which results in the interruption of the release of fed-batch media.
Autoclaving
Before fermentation, it is important to autoclave all media and filter all solutions. It is important to not autoclave some solutions since they have heat labile compounds and hence, they can be added to the autoclaved media later. We filtered, rather than autoclave, the glucose (700 g/l), the trace metal solution (25x), and MgSO4 (245 g/l). Kanamycin was also added later. Further, unlike the paper where they add the antifoam to the media, we used the antifoam sensor of the fermenter to add it when necessary at the default flow rate.
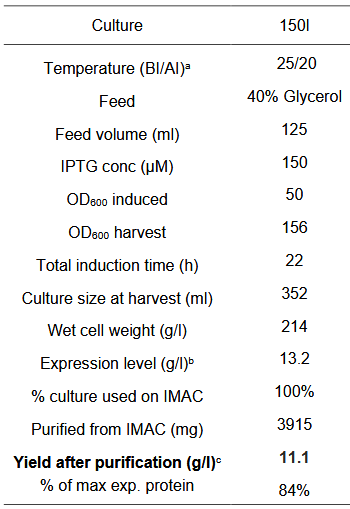
Parameters for the Fermentation cycle
Our fermentation strategy worked in every protein production cycle, but we had to slightly modify the original one (below).
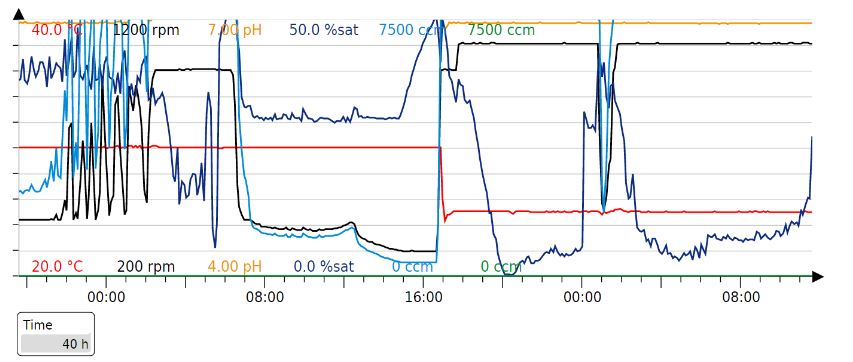
Since glucose inhibits protein expression of T7 expression vectors, the full consumption of glucose has to occur before the beginning of protein expression. Full oxygen consumption is visible as a spike in the saturated oxygen levels (pO2) in the bioreactor trends. In our 3L bioreactor, this tends to happen around 20h and at this point, both induction (150 uM IPTG) and drop in temperature (29->20) should be done. The protein production was stopped after about 20h with a total of 40h of fermentation time per production cycle.
Our production cycle (40h):
For more information, please refer to part BBa_K4247007 (Minispidroin_NT-2rep-CT_N-6His) where the various characterizations we performed on this protein can be found in detail.
LINKS_China 2021 Characterization of CBM3-NT2RepCT-CBM3
Author: Aaron Zhexuan Zhang
Designer: Zixiang Zhou
Part of our project is centered around modifying bacterial cellulose membrane (BCM) produced by Komagataeibacter and Saccharomyces cerevisiae to change BCM's physical properties and to make a leather-like material.
In order to modify BCM’s physical properties, we designed and expressed spider silk fibroins fused with cellulose binding matrixes (CBMs; learn more on our description page) to bind to BCM (Fig. 1). For our project, we experimented with CBM3 from Ruminiclostridium thermocellum (Protein Data Bank (PDB) accession: 1NBC; Fig. 1D) (2) and CBM2 from Cellulomonas fimi (PDB accession: 1EXG; Fig. 1C). For our spider silk protein, we chose to use the synthetic mini spider silk protein NT2RepCT (2Rep; first characterized by GreatBaySZ_2019). 2Rep is water-soluble due to hydrophilic interactions of protein N-terminal and C-terminal. When 2Rep is submerged in a coagulating bath and subjected to a shear force, the repetitive regions will uncoil, form beta-pleated sheet networks and solidify into silk fiber (Fig. 1A).
For adding CBM3 flanking to 2Rep, we synthesized CBM3-BsaI-CBM3 on a pET28a vector. Primers were then used to add BsaI restriction sites in 2Rep to fuse the respective domains together in the synthesized pET28a vector by Golden Gate assembly (Fig. 2A & 2B). After construction, the plasmids were transformed into E. coli BL21(DE3) for IPTG-inducible expression (Fig. 2C).
Comparing the solubility of CBM2/3 fused with 2Rep
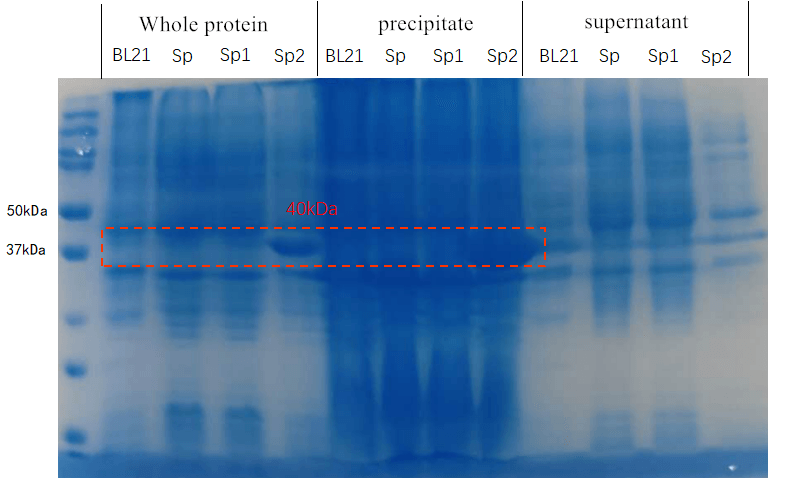
2Rep was shown to possess good water solubility, but we’re uncertain whether our modification will change this desirable property. To test the solubility, we cultured the modified strains, induced the expression of the constructs, collected the cells and performed SDS-PAGE on cell lysate (Fig. 2). Expression of both constructs, CBM3-2Rep-CBM3 (72kDa) and CBM2-2Rep-CBM2 (65kDa), are observed. CBM2-2Rep-CBM2 was present in the whole cell sample, but absent in the cell lysate supernatant, indicating poor water solubility. In contrast, CBM3-2Rep-CBM3 was present in both whole cell and supernatant. Therefore, CBM3-2Rep-CBM3 was chosen for the rest of the project for its superiority in water solubility.

Purification of CBM3-2Rep-CBM3
We purified CBM3-2Rep-CBM3 via the fused his-tag (Fig. 3A), and followed up with BCA assay to measure the yield of our modified spider silk protein. The concentration of CBM3-2Rep-CBM3 is approximately 115.78mg/L (Fig. 3B).
We also constructed a 2Rep plasmid capable of expression in E. coli BL21(DE3) (Fig. 4A). We expressed 2Rep alongside CBM3-2Rep-CBM3 and purified both proteins (Fig. 4B & C). CBM3-2Rep-CBM3 had a concentration of 133.08mg/L, which was higher than 2Rep’s concentration of 98.44mg/L (Fig. 4D). Both proteins demonstrated great water solubility.

Measuring physical properties of BCM mixed with 2Rep and CBM3-2Rep-CBM3
After purification of both 2Rep and CBM3-2Rep-CBM3, we went on to test whether the binding of additional spider silk layer can enhance the ability of BCM enduring pulling and the softness of our material. To bind our protein to BCM and to prevent rehydration of BCM, we added protein elute to dried BCM, soaked BCM in ethanol and subsequently soup water, and dried the BCM.
We compared the maximal force of BCM added with 2Rep or CBM3-2Rep-CBM3 We found statistical significant increase in maximal force endured when BCM mixed with CBM3-2Rep-CBM3.We then compared the effect of adding different quantities of 2Rep and CBM3-2Rep-CBM3 on maximal force of BCM (Fig. 5C & D), and found a one-fold increase from raw BCM to BCM with 5mg 2Rep and 1.5-fold increase from raw BCM to BCM with 5mg CBM3-2Rep-CBM3. We noticed that a statistically significant increase in maximal force endured will only be observed after adding at least 3.4mg of CBM3-2Rep-CBM3.

We then compared the softness of BCM mixed with different quantities of 2Rep and CBM3-2Rep-CBM3 (Fig. 6B & 6C). Both comparisons showed around 35% increase between raw BCM and BCM with 3.4mg of protein. Same amounts of 2Rep and CBM3-2Rep-CBM3 generally achieved similar results, indicating that softness is not affected by the addition of CBM3.
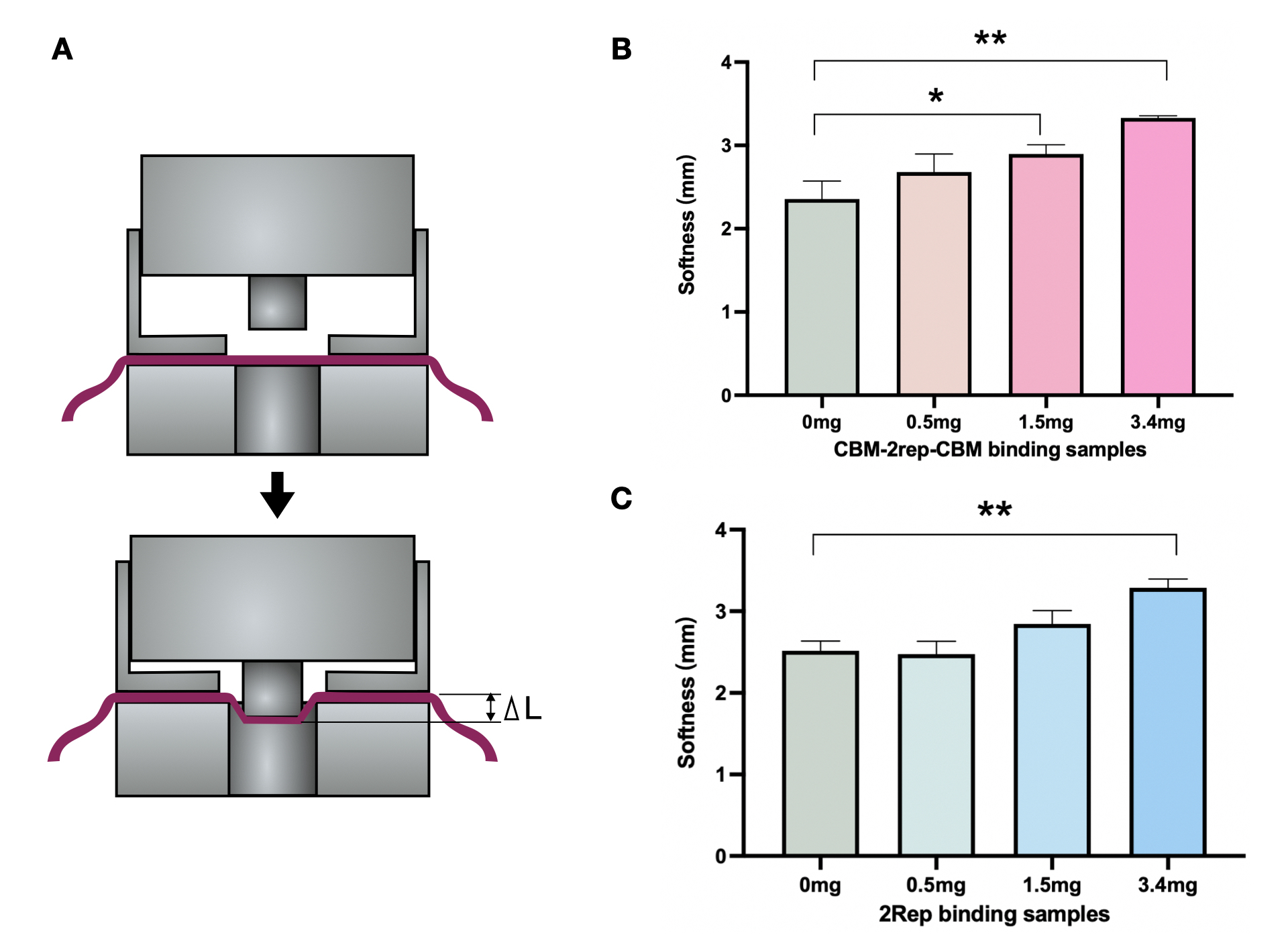
Overall, we have demonstrated that adding 2Rep and CBM3-2Rep-CBM3 can significantly improve the physical characteristics of BCM, with CBM3-2Rep-CBM3 outperforming 2Rep in improved maximal force. This demonstrates the viability of spider-silk-BCM composite material to be used as a leather substitute.
iGEM2020_Nanjing-China Experiment
Group: Nanjing-China 2020
Author: Guangyu Hu
Documentation:
We made some contributions to the expression and purification of spider silk protein gene, that is, we found that the expression level and purifying productivity were low when using PET29a vector.
We attached spider silk protein gene sequence to PET29a vector and obtain the recombinant plasmid PET29a-sp, which was transformed to the competent cell BL21 (DE3) and induced by 1M IPTG at 25℃ overnight for later protein purification. The protein purification result shows that spider silk protein produced by recombinant plasmid PET29a-sp has weaker interaction with Ni column, causing poor purifying results.
In the study from GreatBay SZ, spider silk protein gene sequence was usually attached to PET29a vector. And in the spider silk protein expressed by recombinant plasmid PET28a-sp, there were two 6-residue His tags in both C terminal and N terminal, which could interact with Ni column and help the purification of spider silk protein. However, the spider silk protein expressing by recombinant plasmid PET29a-sp had one 6-residue His tag in C terminal. This difference resulted in the poor purifying results of spider silk protein by using Ni column.
Reference
[1] Andersson, Marlene, et al. “Biomimetic Spinning of Artificial Spider Silk from a Chimeric Minispidroin.” /Nature Chemical Biology/, vol. 13, no. 3, Sept. 2017, pp. 262–264., doi:10.1038/nchembio.2269.
Usage and Biology
Spider silk serves as a new material with superior properties that can be applied in medication, cloth, and aerospace fields. However, spider breeding is not applicable due to spider's fierce behavior. The current approach is to produce recombinant spidroins (silk proteins) from other chassis and spin them into silk.
NT-2Rep-CT is the common architecture in two main types of spider silk proteins: major ambulate spidroins(MaSps) and minor ampullate spidorins (MiSps). NT is a non-repetitive N-terminal domain, as well as CT, the C-terminal domain. In between them is an extensive repetitive region. In the storage of spider silk protein, NT is a monomeric and highly soluble region in neutral pH, and it provides the solubility of the entire spidorin. NT can form stable dimers when pH decreases in the spinning duct. On the other side during the decrease of pH, the CT gets disrupted and unfold to turn in the beta-sheet amyloid-like fibrils. It is hypothesized that structural change of CT precipitate the change of the repetitive region to beta-sheet conformation. Based on the part NT-2Rep-CT, development was carried out by our group to enable a step closer the recently unattainable size of nature spidroin(556 kDa) that contains192 repeat motifs of the Nephila clavipes dragline spidroin. More details please go to NT4RepCT. (Andersson, Marlene, et al. 2017)
Design Considerations
We found the amino acid sequence of NT domain, CT domain and extensive region Rep. Codon optimization was carried out by us to code the nucleotide sequence of NT-2Rep-CT according to the Escherichia coli (E.coli) codon bias. The construct was cloned into a pT7 plasmid and transformed into BL21 (DE3) E. coli. The construction includes:
1. a 6× His tag (MGHHHHHHM) is added to enable us carrying out Ni-NTA protein purification
2. an N-terminal domain based on the E. australis MaSp1 sequence (SHTTPWTNPGLAENFMNSFMQGLSSMPGFTASQLDDM STIAQSMVQSIQSLAAQGRTSPNKLQALNMAFASSMAEIAASEEGGG SLSTKTSSIASAMSNAFLQTTGVVNQPFINEITQLVSMFAQAGMNDVSA; EMBL accession number AM259067)
3. a repetitive part consisting of two polyalanine and glycine-rich repeat regions from MaSp1 of E. australis (GNSGRGQGGYGQGSGGNAAAAAAAAAAAAAAAGQGGQGGYGR QSQGAGSAAAAAAAAAAAAAAGSGQGGYGGQGQGGYGQSGNS; EMBL accession number AJ973155)
4. a C-terminal domain based on the A. ventricosus MiSp sequence (VTSGGYGYGTSAAAGAGVAAGSYAGAVN RLSSAEAASRVSSNIAAIASGGASALPSVISNIYSGVVA SGVSSNEALI QALL ELLSALVHVLSSASIGNVSSVGVDSTLNVVQ DSVGQYVG; GenBank accession number JX513956).
Also, the linker between the NT/CT and the 2Rep(repetitive region) is GNS
Characterization
We aimed to use a synthetic biology approach to combine standardized DNA part NT-2Rep-CT, and transform constructs to metabolically engineered E. coli for bioproduction.
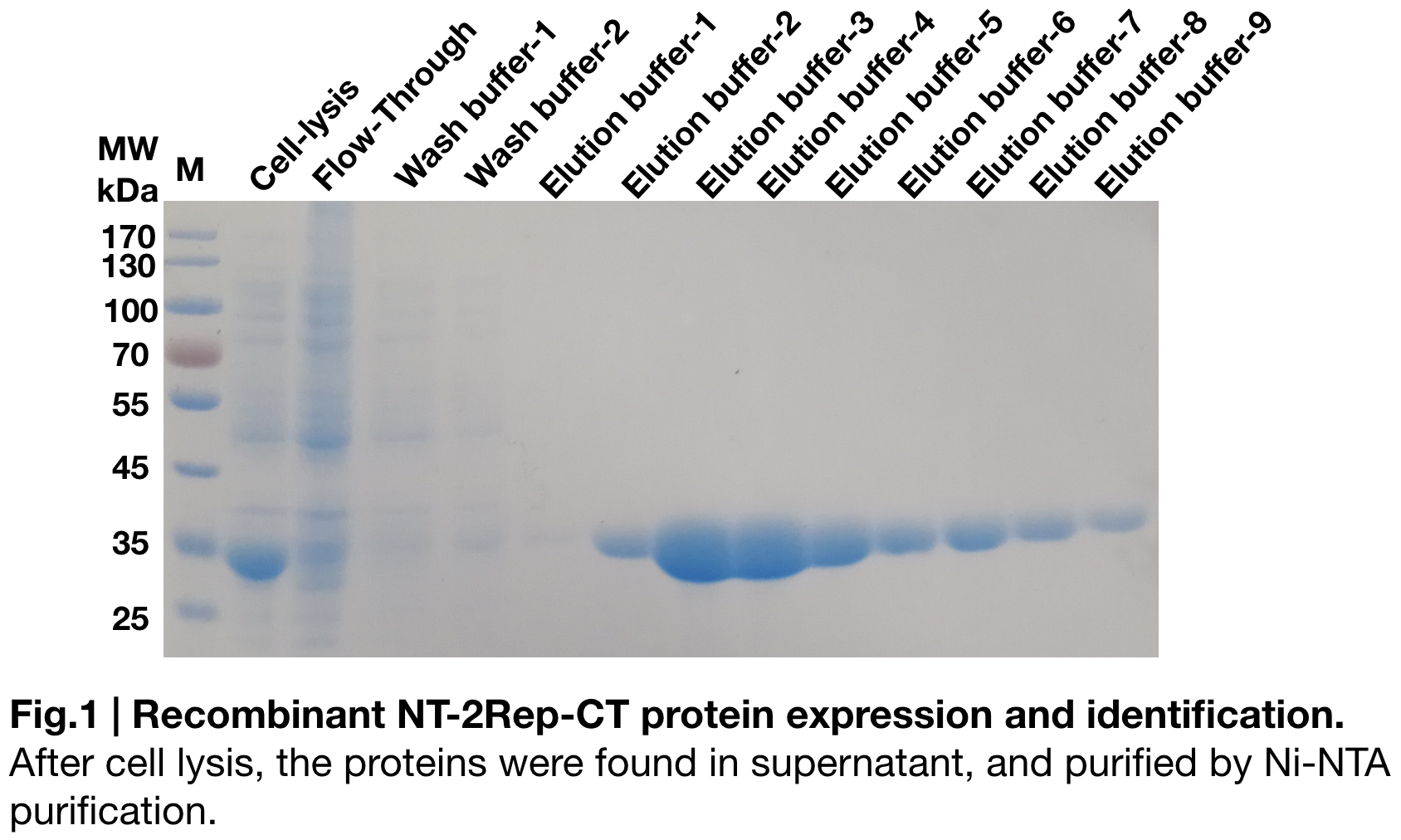
Purification and SDS PAGE
In order to detect whether protein expression was induced by adding isopropylthiogalactoside (final concentration 0.3mM), we used SDS-page(12%) to determine the presence of target protein. The induced protein is 33kDa, which it consistent with the previous research (Andersson, Marlene, et al. 2017).
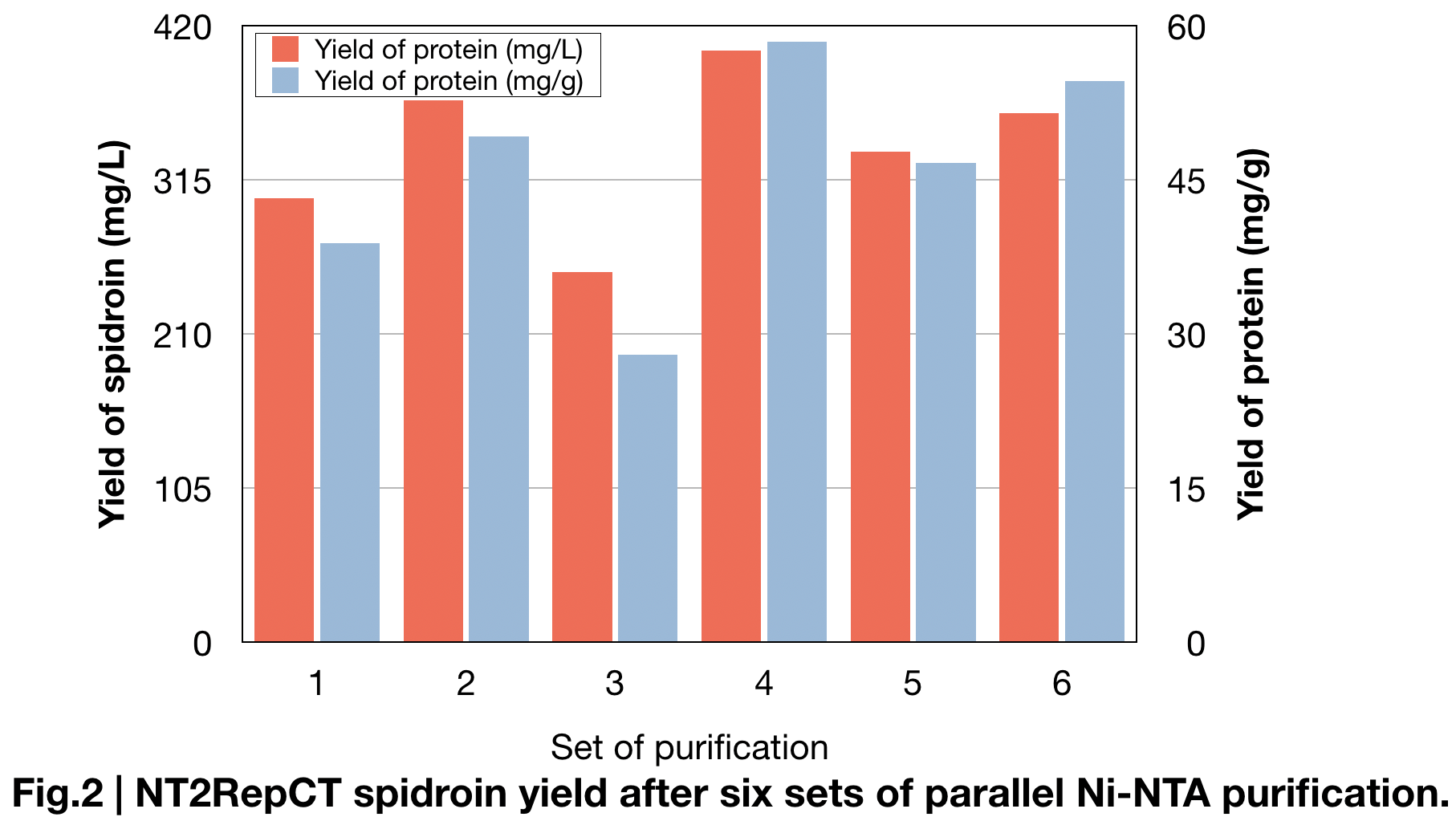
Spidroin Production measure
BCA protein essay kit give us the specified data of the average amount of protein we extracted in parallel sets of Ni-NTA purification (Fig.2). Result shows the amount of spidroin in shaking-flask E.coli cultures and yielded average around 336±49 mg protein/L, and 46±10mg/g, which is more than double of the yield in previous research (125mg protein/L) (Andersson, Marlene, et al. 2017) and most yielded protein among all spidroins we produced this year.
Fiber spinning
NT2RepCT has the feature of pH-dependent assembly, because lowered pH and shearing induce adjusts conformational changes that result in conversion from soluble protein to beta-sheet fibers. At pH>7, NT is in highly soluble state, which lead the spidroin to have high solubility. Due to the lock and trigger mechanism, when pH is lowered in the end of the silk duct, NT forms stable dimers and therefore interconnects the spidroins by applying pulling force alone the fiber via protein chains; CT, at the same time, unfolds to form amyloid-like fibrils that serves as the nucleation seeds of the conversion of the repetitive region (2Rep) into beta-sheet structures. (Andersson, Marlene, et al. 2017)
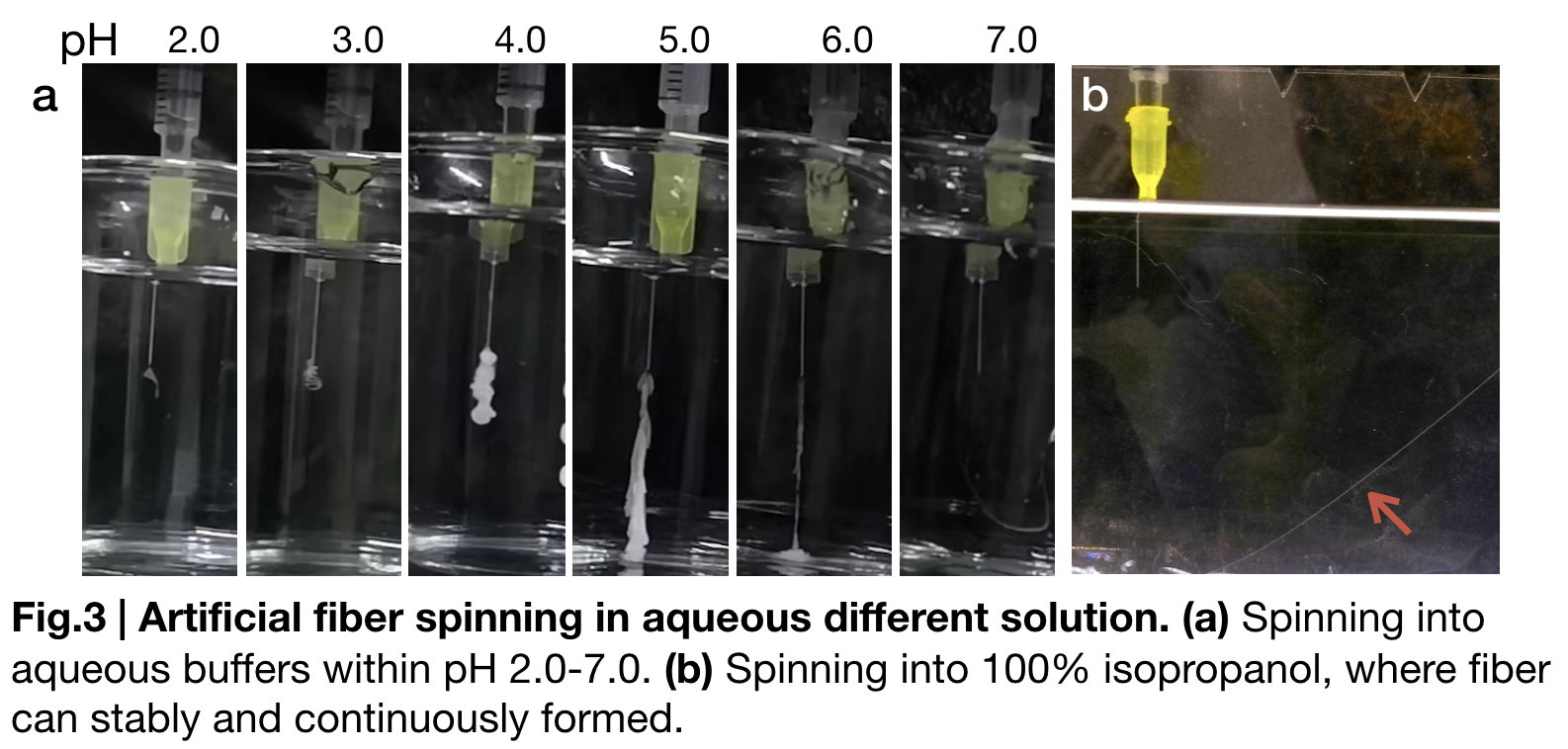
To further substantiate the role of pH in fiber spinning, we carried out artificial fiber spinning in different aqueous buffers that valued from (pH2.0 to pH7.0) with same extruding speed. (Fig.3.a) sketches these relationships. When pH of the buffer bath is from 2.0 to 4.0 gelatinous substance forms and attach to the syringe until disappearing shortly in the solution. Continuous fibers formed when buffer pH is in between 5.0 to 6.0. Fiber is visually continuously formed in pH=7.0 buffer, but disrupted easily when we try to reach the fiber and lift. Besides the pH change, in our biomimetic spinning, we also used 100% isopropanol (Fig.3.b) in the fibre spinning solution and gained a continuous and uniformly-distributed fibre that would not be interrupted by lifting to collect.
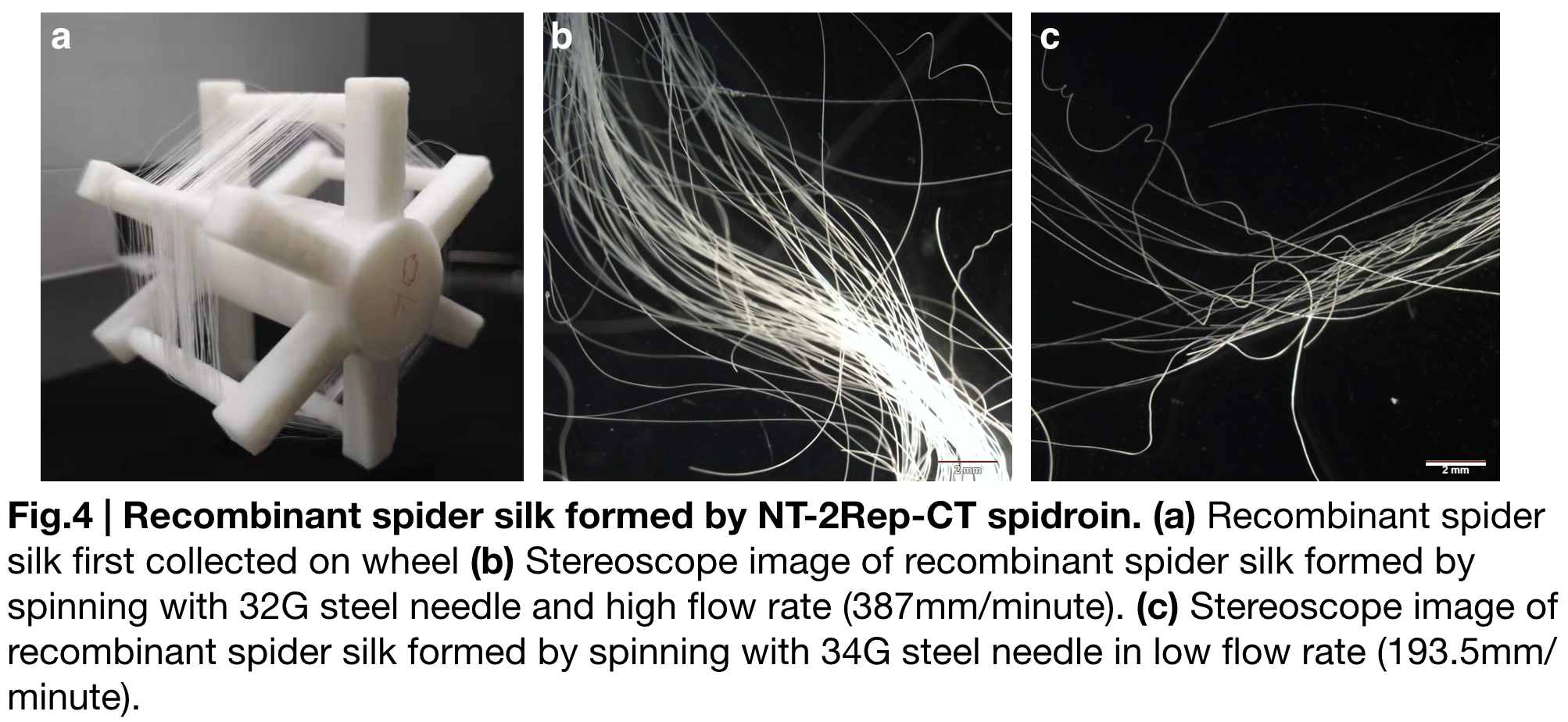
NT-2Rep-CT spidroin formed continuous and stable recombinant silk in our artificial spinning. (Fig.4.a.) To investigate the role of flow rate and tip diameter of the syringe, we artificially spun spidroin NT-2Rep-CT into 100% isopropanol and varied these factors. Stereoscope image was taken for both kind of silk, and showed no difference in their features including diameter or light transmittance. Applying the result to the role of each region of the spidroin, we believe the spider silk property is subjectively caused by solubility of NT, unfolding property of CT and beta-sheet formed by the extensive region Rep.
Scanning electron microscopy of fibers
Aiming to verify the distribution uniformity of NT2RepCT after spinning into fiber, we looked at our artificial spun silks by NT-2Rep-CT spidroin and concocting NT-2Rep-CT spidroin with sfGFP chromoprotein under electron microscope (Fig.5), the role of sfGFP chromoprotein here is for us to clearly observe our silk under electron microscope. We randomly selected the cross section in our continuous silk, it is clearly shown that the fiber circularity is qualified as a uniformly distributed silk. Without sfGFP mixed, the spider silk spun by NT-2Rep-CT spidroin only can not be observed from the positive electron microscope image due to its lack of fluorescence.
Team CNPEM-Brazil 2024 literature review
Our objective is to deliver a comprehensive and updated literature review about the Nt2RepCt, which holds substantial biotechnological potential for diverse applications across multiple fields.
- Coacervate Microdroplets: A study published in June 2023 focuses on the construction of coacervate microdroplets using recombinant spidroin NT2RepCT. This research emphasizes the ability of these microdroplets to respond to environmental stimuli, making them suitable for simulating advanced life behaviors. The morphology of these droplets can be manipulated by altering conditions such as protein concentration, pH, and temperature, which is crucial for understanding material properties and phase behavior in artificial cell systems. [1]
- Nt2RepCt hydrogel: This study from May, 2023 discusses the innovative development of hydrogels derived from recombinant spider silk proteins, which can be tailored for specific biomedical applications. The researchers demonstrate how these hydrogels can be engineered to possess adjustable mechanical and physical properties, making them suitable for controlled drug release and 3D cell culture environments. The study emphasizes the potential of these spider silk-based hydrogels to mimic the extracellular matrix, thereby enhancing cell growth and interaction. This work highlights the versatility and biocompatibility of spider silk proteins, positioning them as promising materials for future applications in tissue engineering and regenerative medicine. [2]
- Synthetic spider silk: A study published in July, 2024 addresses the challenge of replicating the exceptional mechanical properties of natural spider silk through synthetic methods. It introduces a novel approach using multiarm polyethylene glycol (PEG) to create synthetic spider silk that retains biomimetic qualities while enhancing tensile strength and extensibility. The study highlights the versatility of bioconjugation techniques in modifying protein structures, facilitating spidroin crosslinking, and chemical functionalization. By employing this method, the researchers aim to improve the toughness of the fibers without needing to develop new mini-spidroin constructs, thus potentially mitigating the adverse effects of environmental conditions on synthetic spider silk applications. [3]
- Studying structural variants: The study from september, 2023, investigates the role of a spider silk protein, NT2RepCT, in promoting liquid-liquid phase separation, a critical process in the formation of intracellular condensates within yeast cells (Saccharomyces cerevisiae). The study explores how different structural variants of NT2RepCT behave under varying intracellular conditions, revealing that factors such as molecular crowding and pH significantly influence the condensation properties and growth of these protein assemblies. By conducting parallel in vivo and in vitro experiments, the researchers demonstrate that yeast cells can provide a unique environment for studying protein condensation mechanisms, which could inform future designs of biomolecular materials for synthetic biology applications. [4]