Difference between revisions of "Part:BBa K4202045"
(→Result) |
|||
| Line 8: | Line 8: | ||
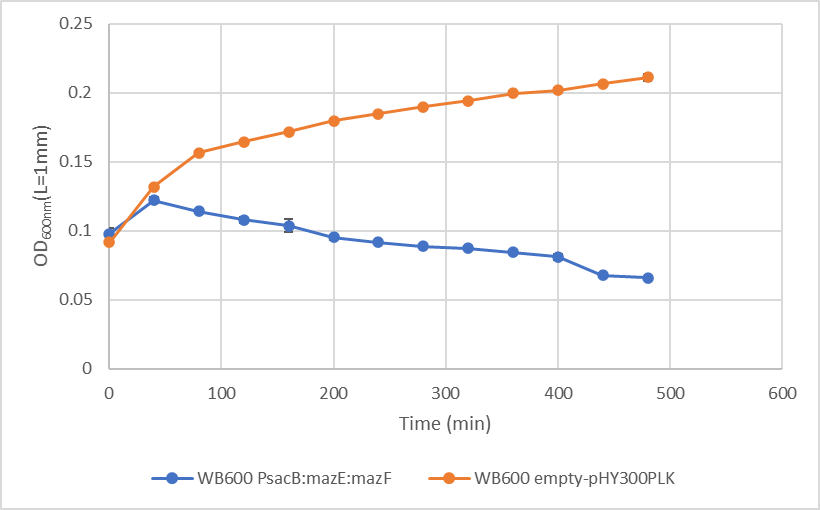
<p>To characterized our kill switch, we transformed both the empty vector and the vector containing the kill switch into Bacillus subtilis WB600 and cultured them in the LB medium containing 1% sucrose. The OD600nm(L=1mm) of the cultures was measured every 40 minutes when the OD600nm(L=1mm) of cultures reached approximately 0.1.</p> | <p>To characterized our kill switch, we transformed both the empty vector and the vector containing the kill switch into Bacillus subtilis WB600 and cultured them in the LB medium containing 1% sucrose. The OD600nm(L=1mm) of the cultures was measured every 40 minutes when the OD600nm(L=1mm) of cultures reached approximately 0.1.</p> | ||
<p>Compared to the control group, the OD<sub>600nm</sub>(L=1mm) of strains containing the kill switch began to decrease after the <sub>600nm</sub>(L=1mm) reached 0.12. The early decrease of OD<sub>600nm</sub> indicated that the bacteria are dying, which means our kill switch is working.</p> | <p>Compared to the control group, the OD<sub>600nm</sub>(L=1mm) of strains containing the kill switch began to decrease after the <sub>600nm</sub>(L=1mm) reached 0.12. The early decrease of OD<sub>600nm</sub> indicated that the bacteria are dying, which means our kill switch is working.</p> | ||
| − | [[File:Kill result.png]] | + | <div align="center">[[File:Kill result.png|500px]]</div> |
| − | <div align="center" | + | <div align="center"><b>Fig 1</b>The growth curve of two <i>Bacillus subtilis</i> WB600 strains.</div> <div align="center"><p>Blue curve represents strains containing suicide switches and the yellow line represents strains containing the empty plasmid(CK)</p></div> |
| − | + | <br> | |
===Usage and Biology=== | ===Usage and Biology=== | ||
==Biology== | ==Biology== | ||
Latest revision as of 11:27, 12 October 2022
Sucrose regulated kill switch in Bacillus subtilis
Sucrose-induced promoters are linked to the toxin/antitoxin system. When the sucrose concentration is high enough, the promoter is efficient, and MazE continuously expresses the antagonistic toxin MazF; When the sucrose concentration is insufficient, the promoter efficiency is insufficient, MazE cannot be expressed continuously, and based on its instability, its cell concentration will decrease faster than MazF, causing MazF to exert its toxic effects and eventually lead to cell death. This part prevents engineering bacteria from escaping into the environment under certain stressful conditions.
Result
To characterized our kill switch, we transformed both the empty vector and the vector containing the kill switch into Bacillus subtilis WB600 and cultured them in the LB medium containing 1% sucrose. The OD600nm(L=1mm) of the cultures was measured every 40 minutes when the OD600nm(L=1mm) of cultures reached approximately 0.1.
Compared to the control group, the OD600nm(L=1mm) of strains containing the kill switch began to decrease after the 600nm(L=1mm) reached 0.12. The early decrease of OD600nm indicated that the bacteria are dying, which means our kill switch is working.
Blue curve represents strains containing suicide switches and the yellow line represents strains containing the empty plasmid(CK)
Usage and Biology
Biology
The positive regulatory promoter PsacB is a weak endogenous promoter and can be induced by the sucrose [1]. It contains two parts, the core constitutive promoter and the leader RNA sequence. The leader RNA sequence contains a terminator overlaping with the binding site of sacY [2] [3]. When the sucrose existed, the sacY can bind to the bing site in the leader RNA and stablized the terminator, which enables the transcription. The absence of sucrose can lead to the formation of terminator structure, which will lead to the early termination of downstream genes. The antitoxin-toxin system is consisted by the coding sequence mazE and mazF. The mazF sequence used in our project is from E. coli and can degrade the mRNA nonspecificly. The antitoxin mazE can bind to the mazF and inhibit the activity of mazF.
Reference
[1] Aymerich, S., Gonzy-Tréboul, G., & Steinmetz, M. (1986). 5'-Noncoding region SACR is the target of all identified regulation affecting the LEVANSUCRASE gene in bacillus subtilis. Journal of Bacteriology, 166(3), 993–998. https://doi.org/10.1128/jb.166.3.993-998.1986
[2] Tortosa, P., & Le Coq, D. (1995). A ribonucleic antiterminator sequence (rat) and a distant palindrome are both involved in sucrose induction of the bacillus subtilis sacxy regulatory operon. Microbiology, 141(11), 2921–2927. https://doi.org/10.1099/13500872-141-11-2921
[3] Clerte, C., Declerck, N., & Margeat, E. (2013). Competitive folding of anti-terminator/terminator hairpins monitored by Single Molecule Fret. Nucleic Acids Research, 41(4), 2632–2643. https://doi.org/10.1093/nar/gks1315
Usage
Over the past decade, PsacB has been widely used as one of the few inducible promoters in Bacillus subtilis for protein expression, secondary metabolite production, and synthetic biology research. The mazE-mazF antitoxin-toxin system from E. coli has been widely used for density regulation of engineered bacterial populations and the construction of kill switches. In our project, the PsacB promoter was used together with the mazE-mazF antitoxin-toxin system to construct a kill switch for engineered Bacillus subtilis to commit suicide at a later stage of bacterial growth.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 926
Illegal AgeI site found at 654 - 1000INCOMPATIBLE WITH RFC[1000]Illegal SapI.rc site found at 1139