Difference between revisions of "Part:BBa K4274000"
| Line 29: | Line 29: | ||
<br>Afterwards, the various engineering of <i>E.coli</i> DH5α ∆TnaA mentioned above were used for santalene production. After rapid centrifugation, the super | <br>Afterwards, the various engineering of <i>E.coli</i> DH5α ∆TnaA mentioned above were used for santalene production. After rapid centrifugation, the super | ||
| + | ==Sequence and features== | ||
<partinfo>BBa_K4274000 SequenceAndFeatures</partinfo> | <partinfo>BBa_K4274000 SequenceAndFeatures</partinfo> | ||
Revision as of 09:04, 12 October 2022
ClSS_S533A
ClSS_S533A is an optimized biobrick part encoding the gene for alpha-santalene synthase from Clausena lansium. The enzyme catalyzes the conversion of the common isoprenoid intermediate farnesyl pyrophosphate (FPP) into the alpha-santelene in a single step. It is reported that ClSS's basic amino acid residue S533’s mutation to typical nonpolar amino acid Ala could result in a 1.7-fold increase in production of alpha-santalene compared to the nonmutated strain (Jia Z.et al., 2022) .
This year, we are using ClSS_S533A to construct composite parts ptac-RiboJ-B0034-Cl</i>SS_S533A-B0034-ERG20-B0015 (part: BBa_K4274020), ptac-RiboJ-B0034-ClSS_S533A-B0034-ERG20_F96W-B0015 (part: BBa_K4274021) and ptac-RiboJ-B0034-ClSS_S533A-FL-ERG20_F96W-B0015 (part: BBa_K4274023). This will allow engineering E.coli DH5a (tnaA-) to heterologously express ClSS_S533A. Other teams can utilize this part for E. coli alpha-santalene production.
Usage and Biology
ClSS_S533A is an optimized biobrick part encoding the gene for alpha-santalene synthase from Clausena lansium. It was firstly characterized by Jia Z.in 2022, who mutated ClSS's basic amino acid residue S533 to increase the production of alpha-santalene in E. coli (Jia Z.et al., 2022).
Natural ClSS is an alpha-santalene synthase which could catalyze the conversion of the common isoprenoid intermediate farnesyl pyrophosphate (FPP) into the alpha-santelene in a single step. It has been successfully heterologously expressed to produce functional terpene product in both yeast (Wenlong Z.et al., 2020) and E. coli (Jia Z.et al., 2022). But the mutation of residue S533 to Ala led to the addition of two hydrogen bonds near this site (<4 Å), which resulted in a high α-santalene production E.coli strain.
In our study, ClSS_S533A could be heterologously expressed in E. coli, and allows for fused proteins to be bound to ERG20 (Part: BBa_K849001) and ERG20_F96W (Part: BBa_K4274002)
Source
Clausena lansium
Characterization
After engineering, E. coli could utilize both MEP pathway and MVA pathway for the universal precursors isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP), then synthesize santalene with the help of FPP Synthase (FPPS) and santalene synthase (SS). Except heterologously expressed MVA pathway and ERG20 of Saccharomyces cerevisiae and santalene synthase of Clausena lansium (ClSS), several modifications upon ERG20 or ClSS by amino acid mutation, binding to a hydrophillic tag and the construction of fusion protein were tested for the higher yield of santalene. Therefore, with the help of the co-transformation of pMVA plasmid with various pW1 plasmids, including pW1_CE, pW1_CEM, pW1_TCEM and pW1_CEM_FL, different strains like CE, CEM, TCEM, CEM_FL were successfully constructed (Figure 1). The complete pathway we designed for producing santalene in E. coli is illustrated in Figure 1.
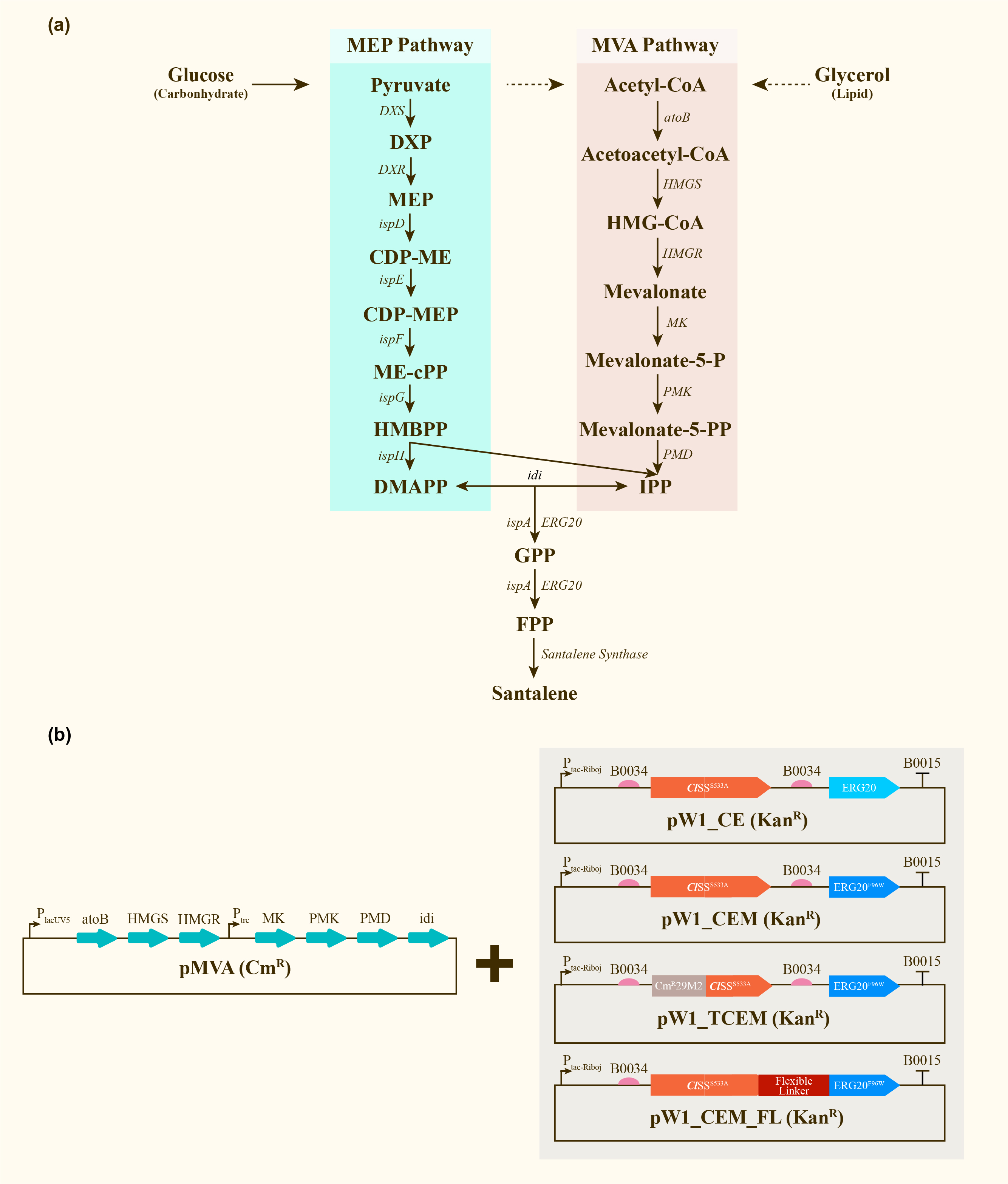
Afterwards, the various engineering of E.coli DH5α ∆TnaA mentioned above were used for santalene production. After rapid centrifugation, the super
Sequence and features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 183
- 1000COMPATIBLE WITH RFC[1000]
