Difference between revisions of "Part:BBa J428351:Experience"
| Line 55: | Line 55: | ||
======Prize Data====== | ======Prize Data====== | ||
| − | [[File:PSC101 Characterisation - 220906 | + | [[File:PSC101 Characterisation - 220906 FI Plot per DV.png|400px|thumb|left| |
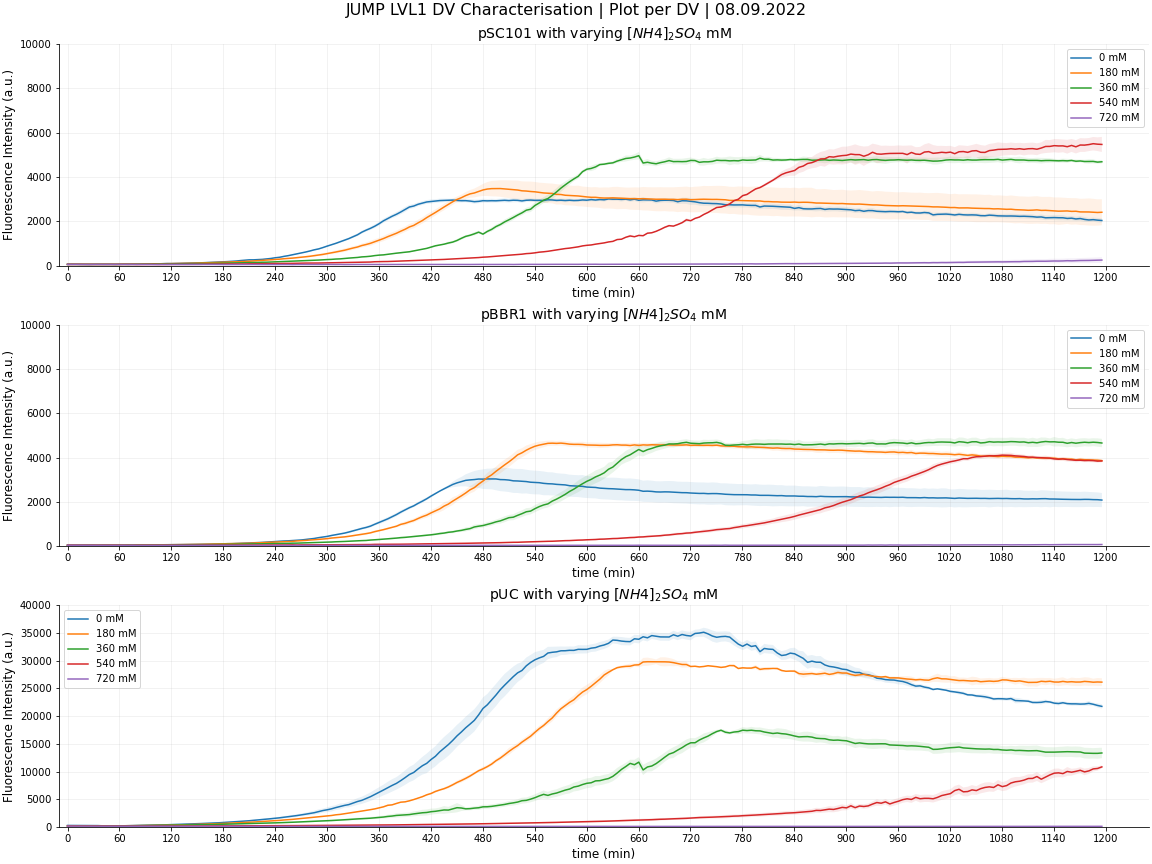
<center>'''Figure 3: Fluorescence Intensity of JUMP DVs with varying [NH4]2SO4 mM'''</center> | <center>'''Figure 3: Fluorescence Intensity of JUMP DVs with varying [NH4]2SO4 mM'''</center> | ||
<p> | <p> | ||
| Line 79: | Line 79: | ||
<li>DV pUC (high copy no.) : For high copy number plasmids, we can report that any ammonium concentration is detrimental to the cells expression. The greatest fluorescence was recorded with 0mM of ammonium sulphate, with decreasing expression as it was increased. | <li>DV pUC (high copy no.) : For high copy number plasmids, we can report that any ammonium concentration is detrimental to the cells expression. The greatest fluorescence was recorded with 0mM of ammonium sulphate, with decreasing expression as it was increased. | ||
| + | <br><br><br><br><br> | ||
======Growth (OD600)====== | ======Growth (OD600)====== | ||
| − | + | However, we did not observe the same scenario with growth. In fact, we found that ANY concentration of ammonium was detrimental to the cells, regardless of the copy number plasmid it contained. | |
This means that if you were to use pSC101 with 540mM of ammonium to maximise protein expression, you will have to compromise on the growth rate. | This means that if you were to use pSC101 with 540mM of ammonium to maximise protein expression, you will have to compromise on the growth rate. | ||
| + | |||
| + | [[File:PSC101 Characterisation - 220906 OD600 Plot per DV.png|400px|thumb|right| | ||
| + | <center>'''Figure 4: Growth of JUMP DVs with varying [NH4]2SO4 mM'''</center> | ||
| + | <p> | ||
| + | Ammonium sulphate concentration: 0mM-1000mM | ||
| + | </p>''' ]] | ||
| + | |||
[[File:PSC101 Characterisation - 220908 OD600 Plot per DV.png|400px|thumb|right| | [[File:PSC101 Characterisation - 220908 OD600 Plot per DV.png|400px|thumb|right| | ||
| − | <center>'''Figure | + | <center>'''Figure 5: Growth of JUMP DVs with varying [NH4]2SO4 mM'''</center> |
<p> | <p> | ||
Ammonium sulphate concentration: 0mM-720mM | Ammonium sulphate concentration: 0mM-720mM | ||
</p>''' ]] | </p>''' ]] | ||
| − | + | <br><br><br><br> | |
===User Reviews=== | ===User Reviews=== | ||
<!-- DON'T DELETE --><partinfo>BBa_J428351 StartReviews</partinfo> | <!-- DON'T DELETE --><partinfo>BBa_J428351 StartReviews</partinfo> | ||
Revision as of 21:36, 11 October 2022
This experience page is provided so that any user may enter their experience using this part.
Please enter
how you used this part and how it worked out.
Applications of BBa_J428351
Cambridge 2022 JUMP DV Characterisation
Experimental approach
Why Ammonium?
Our iGEM project this year involves perturbations that cells can be subjected to. We are creating an 'antithetic integral controller' used to ensure that protein expression remains at a stable, desired level in the presence of conditions that could perturb it. During one of our Integrated Human Practice discussions with protein engineering company, Codexis, the Cambridge team discussed common perturbations that cells are likely to be subjected to in industry, where we were informed about ammonium build up in bioreactors in the case of insufficient mixing. We thought that we could then use this to access the effect of ammonium on different copy number plasmids and see whether they would respond differently to each other.
JUMP DV Considerations
Out of the 3 JUMP DVs that we characterised in E. coli, we aim to focus on pSC101 (low copy no.) as its growth, compared to the other 2 DVs, was less sporadic and didn't go through such a dramatic death phase, instead remaining at the stationary phase, once it had finished growing, until the end of our experiment. Low copy number plasmids are also often used as a default as they exert less burden on cells so this allows us to made a larger contribution to the iGEM and synthetic biology community. pSC101 being a low-copy number plasmid, will therefore exert less burden on our cells - this is in particular important for the construction and functionality of the complex 'robust perfect adaptation' circuit.
Experimental Setup & Protocol
We used a 96- well plate in the Clariostar Plate reader, changing the ammonium sulphate concentration in the growth media and the copy number plasmid in DH5a E. coli:
Liquid Culture Preparation
- Label 5 snap caps with plasmid/sample name: ‘just cells’, ‘lvl 1’, ‘pSC101’, ‘pBBR1’, ‘pUC’
- 2mL of EZRDM added to all 5
- In all but ‘just cells’, 2uL of Kan antibiotic is added
- Innoculate liquid culture
- Put snapcaps in shaking incubator for 24 hours at 30 degrees at 180rpm
Pippetting the well plate
- Make 720mM (filter sterilised) stock:
14.4mL of 1000mM [NH4]2SO4 & 5.6mL EZRDM (0mM) =20mL - Label 5 snapcaps with the 5 ammonium sulphate concentrations:
720mM: 4mL 720mM ([NH4]2SO4)
540mM: 3mL 720mM ([NH4]2SO4) & 1mL 0mM (EZRDM)
360mM: 2mL 720mM ([NH4]2SO4) & 2mL 0mM (EZRDM)
180mM: 1mL 720mM ([NH4]2SO4) & 3mL 0mM (EZRDM)
0mM: 750mM: 4mL 0mM (EZRDM) - Pipette up 10mL of [NH4]2SO4 using stripette and release 4ml,3ml,2ml,1ml into 720mM, 540mM, 360mM, 180mM. (leave 0mM empty)
- Then pipette up 10mL of EZRDM using stripette and release 4ml,3ml,2ml,1ml into 0mM, 180mM, 360mM, 540mM. (leave 720mM alone)
- They should all have final vol. of 4mL
- Get red pen to mark of wells I’ve added ammonium sulphate sol. to
- Get green pen to mark when cells have been added.
- ‘Just cells’ will be the only wells with things in without Kanamycin. So pipette 3x 200uL of each stock (into the wells with their replicates:
‘Just cells’ = 0,0,0,180,180,180,360,360,360,540,540,540,720,720,720.) - All the stock now need Kanamycin added so 600uL is gone from each, leaving 3400uL left.
- Add 3.4uL Kan to all the 5 stocks - our just EZRDM with diff ammonium sulphate conc.s wells will also have Kanamycin
- Pipette 200uL of the respective stocks into their wells
- Pipette 1uL of the respective cells into their wells.
- Put transparent film on top of well plate and put in plate reader.
Results and Discussion
Prize Data
We ran our first experiment using ammonium sulphate concentrations of 0mM, 250mM, 500mM, 750mM and 1000mM after reading ‘Ammonium Toxicity in Bacteria’ by Müller et al., 2005, and finding this a reasonable range in order to gather data within and beyond the scope of their testing parameters. The paper claims that ‘in E. coli, addition of 750 mM and 1000 mM ammonium (375 mM and 500 mM (NH4)2SO4) impaired growth’ and with 0-1000mM ammonium sulphate, we aimed to record all stages of detriment to the point at which the bacteria are no longer able to cope.
After running this experiment we confirmed that at 750mM ammonium sulphate, the cells were unable to grow at all so we narrowed down our parameters for the 2nd test. Still using 5 concentrations, this allowed us to interpret more precise details about each plasmid's limits.
Growth (OD600)
However, we did not observe the same scenario with growth. In fact, we found that ANY concentration of ammonium was detrimental to the cells, regardless of the copy number plasmid it contained. This means that if you were to use pSC101 with 540mM of ammonium to maximise protein expression, you will have to compromise on the growth rate.
User Reviews
UNIQf2fc875bead16052-partinfo-00000000-QINU
UNIQf2fc875bead16052-partinfo-00000001-QINU