Difference between revisions of "Part:BBa I719005"
| Line 154: | Line 154: | ||
==II Reference== | ==II Reference== | ||
| − | + | lxs123456 | |
Revision as of 10:39, 30 September 2022
T7 Promoter
Constitutive promoter derived from the T7 bacteriophage. Allows high expression of proteins only when the T7 polymerase is present. This part is identical to the part BBa_R0085 which currently hasn't been built.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
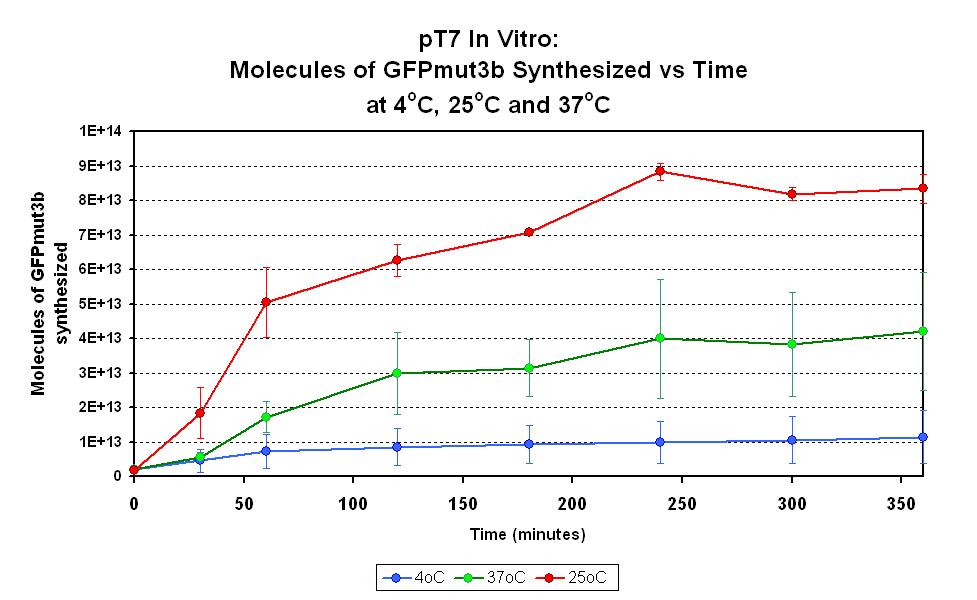
Characterization in vitro
This part has been characterized for temperature sensitivity in the Cell-Free Chassis by iGEM Imperial 2007. For more detail, please check the testing [http://2007.igem.org/Imperial/Wet_Lab/Protocols/Prot1.9 protocols]and [http://2007.igem.org/Imperial/Wet_Lab/Results/Res1.9 results].
| Parameter | Value and Description |
|---|---|
| Optimum temperature | The T7 promoter has a highest output at 25°C, with a one-fold increase in GFP molecules synthesized compared to 37°C. The T7 promoter also has a minimal amount of output at 4°C. |
| Expression Life-span | The rate of GFP synthesis by the T7 promoter reaches a peak at around 30-60 minutes. |
| Peak Expression | The production of GFP decreases to minimal levels after 2 hours and tends towards nil after 4 hours. |
Team Warsaw 2010's measurement
Absolute promoter strength: 41,8pg RNA/minute/ug substrate DNA. It equals 8,92 microPoPSContribution
Group: Valencia_UPV iGEM 2018
Author: Adrián Requena Gutiérrez, Carolina Ropero
Summary: We adapted the part to be able to assemble transcriptional units with the Golden Gate assembly method
Documentation:
In order to create our complete [http://2018.igem.org/Team:Valencia_UPV/Part_Collection part collection] of parts compatible with the Golden Gate assembly method, we made the part BBa_K2656000 which is this part adapted to the Golden Gate technology.
St_Andrews 2019 characterization
We characterised this part using an in vitro T7 polymerase transcription system, measuring the concentration of Firefly Luciferase RNA transcript at 3 different temperatures (30°C, 37°C, 45°C), produced over time. Detail of the protocol can be found on our Experimentspage.
We fitted our results using a beta regression in R, with p << 0.01 for all models. Our results are plotted below. The regressed model is shown by a bold line and a 95% confidence interval is bounded by two dashed lines.
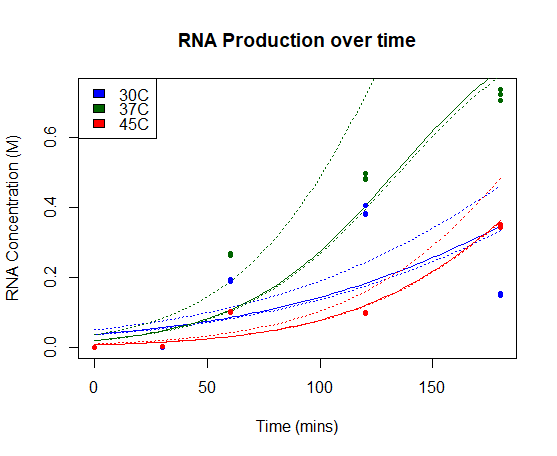
RNA Production over time: Concentration of Firefly Luciferase RNA transcript produced using T7 Polymerase and BBa_I719005 in vitro over time. Models of RNA as a function of time are shown in bold line, with 95% confidence intervals for each model bounded by two dashed lines.
Our results show a highest expression rate of transcript at 37oC when using T7 polymerase in vitro.
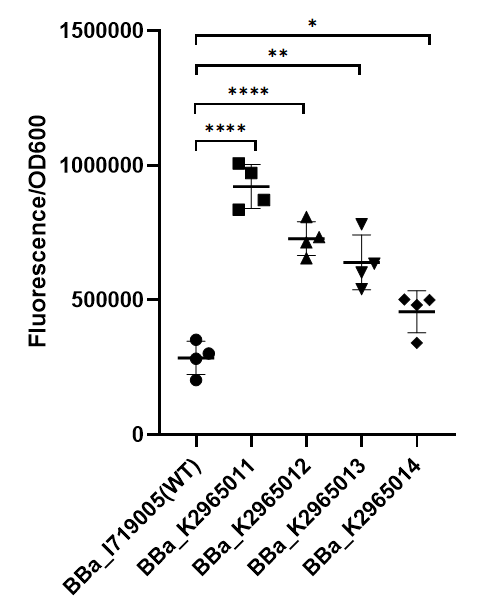
ZJU-China 2019's improvement
I Overview
This year team ZJU-China has upgraded four modified T7 promoters with increased strength (BBa_K2965011, BBa_K2965012, BBa_K2965013, BBa_K2965014). With the help of previous research[1-2], we carefully chose the site which would be mutated by PCR. These sites distribute in the range from -3 to +6. We mutated these sites by adding variable bases to primers (taatacgactcactatagggaga → taatacgactcacWNNNgSRRNN), and screened for stronger mutants. For details, please refer to https://2019.igem.org/Team:ZJU-China/Improve.
II Result
To test the function of mutant promoters, we chose eGFP as our reporter, and added a lac operator behind the promotor to control transcription starts simultaneously. When the E.coli BL21(DE3) is cultured at the stage of logarithmic phase, we added 0.5 mM IPTG to induce the expression of GFP in strains BL21(DE3) for 4 hours. By assessing the absolute fluorescence and OD600, we can conclude the relative strength of all promoters. We screened out four mutants with higher intensity.
As we can see from the figure, our mutant promoters showed largely increased strength compared with wild type T7 promoter.Therefore, our mutant promoters offer users more opportunity to control the expression of protein using T7 promoter and permit higher levels of target protein expression to be obtained.
III Protocol
1. Use BamHI and XhoI to insert eGFP fragment into the pET28 plasmid.
2. Linearize pET28-eGFP plasmid by PCR. (WT: F-CCCGCTGCAGTAATACGACTCACTATAGGGAGAGGAATTGTGAGCGGATAACAA / R- GCGGTGGACTGCAGCAACTCAGCTTCCTTTCGGGCT ; Mu: F-CCCGCTGCAGTAATACGACTCACWNNNGSRRNNGGAATTGTGAGCGGATAACAA / R- GCGGTGGACTGCAGCAACTCAGCTTCCTTTCGGGCT )
3. Cyclization the plasmid by PstI and ligase.
4. Transform the plasmids into E. coli BL21(DE3), add 20μl 100mM IPTG to the plate before coating the plate.
5. Incubate at 37℃ and observe colony color to screen for strong mutants preliminarily.
6. Pick greener single colonies, add the colonies into 3ml Lb, incubate at 37℃ in a shaker for 6-8h to dilute the IPTG introduced when picking single colonies.
7. Add 10μl germ solution from last step into 3ml Lb, incubate at 37℃ in a shaker until OD600 reaches 0.6.
8. Add IPTG to final concentrations 0.5mM, and induce for 4h at 18℃.
9. Measure the fluorescence (excitation wavelength: 485 nm; detection wavelength: 528 nm) and OD600.
IV Reference
[1] Ikeda R A, Ligman C M, Warshamana S, et al. T7 promoter contacts essential for promoter activity in vivo[J]. Nucleic Acids Research, 1992, 20(10): 2517-2524. [2] Paul S, Stang A, Lennartz K, Tenbusch M, Uberla K. Selection of a T7 promoter mutant with enhanced in vitro activity by a novel multi-copy bead display approach for in vitro evolution[J]. Nucleic Acids Research, 2013, 41(1):e29.
SUSTech_Shenzhen 2020 characterization
Protein anti-CRISPR-associated 1 (Aca1) is expressed by T7 promoter, Aca1 will subsequently inhibit the anti-CRISPR promoter, so the gene expression downstream anti-CRISPR will be inhibited until Navitoclax (a compound that can inhibit Aca1 found by SUSTech_Shenzhen) is added artificially. Without the inhibition from Aca1, the expression of genes downstream anti-CRISPR promoter will increase significantly. Therefore, protein expression can be regulated artificially.
We characterised the efficiency of this part of promoting inhibitor in a protein regulation system, the inhibitor was sufficiently expressed to inhibite a downstream promoter, T7 promoter successfully worked in our composite part BBa_K3423005. Green fluorescent protein is used as a reporter, which can reflect the promotion effciency of Aca1 using T7 promoter:

SZ-SHD 2021's characterization
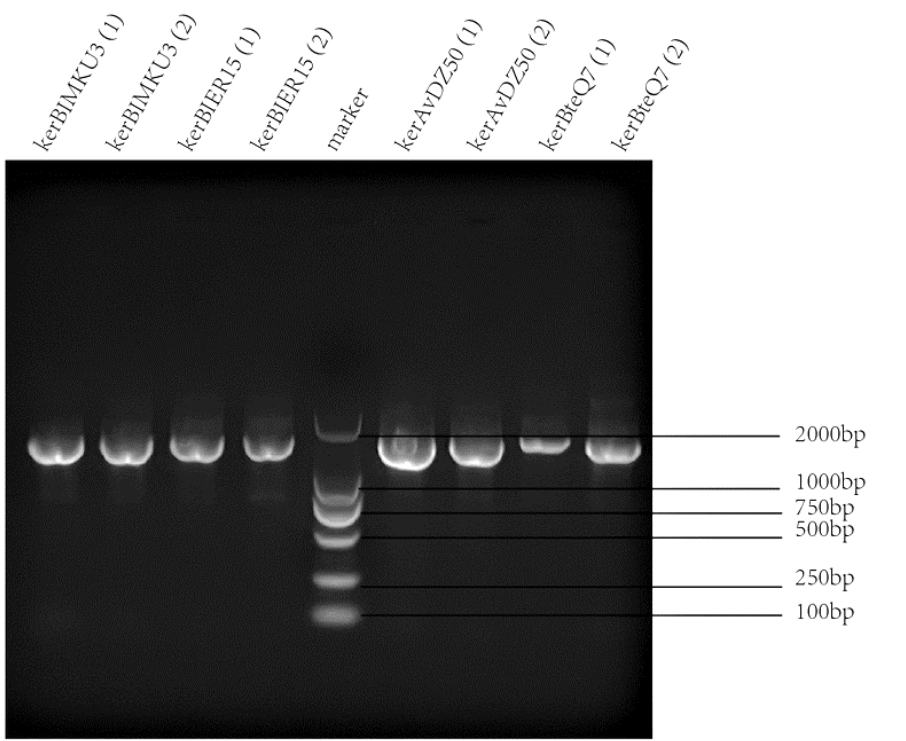
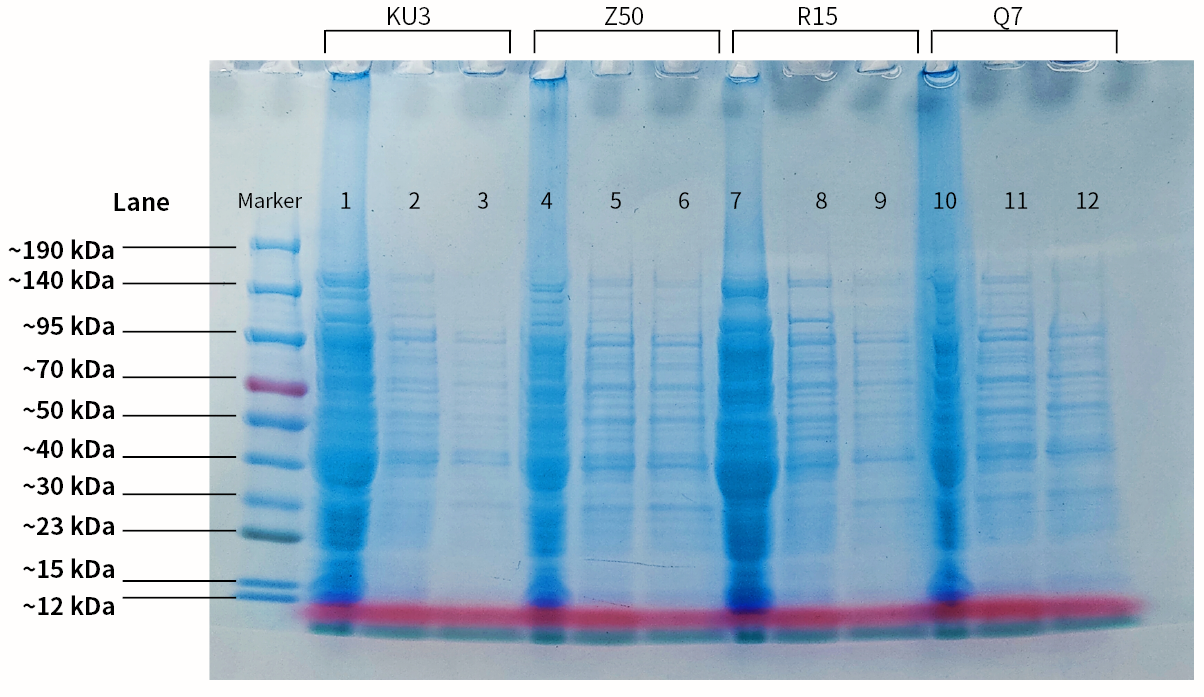
Keratinases kerAvDZ50 (BBa_K3895007), kerBteQ7 (BBa_K3895008), kerBlMKU3 (BBa_K3895009), and kerBlER-15 (BBa_K3895010) were expressed by T7 promoter. The sequences were constructed into PET 28a(+) plasmids and transformed to E.coli BL21 (DE3).
PCR was conducted first for verification of the correct plasmids. Then the amplified DNAs were sequenced (forward primer: TCGATCCCGCGAAATTAATACG; reverse primer: AGGGGTTATGCTAGTTATTGCTCA) and compared with the designed DNA sequences for further verification.
| Name of keratinase | IPTG concentration/mM | Temperature/°C | Time/h |
|---|---|---|---|
| KerBteQ7 | 5 | 16 | 12 |
| KerAVDZ50 | 0.67139 | 16 | 12 |
| KerBIER15 | 0.4196 | 37 | 16 |
| KerBIMKU3 | 0.4 | 37 | 4 |
SDSZ_China 2021 contribution
T7 promoter is commonly used in iGEM projects, in our experiment, LacI was designed as a repressor and IPTG as an inducer to control the state of T7 promoter. To verify its function, we incorporated T7 promoter with LacI operon on pET28b backbone. Then the plasmid was transformed to E.coli top10 for test.
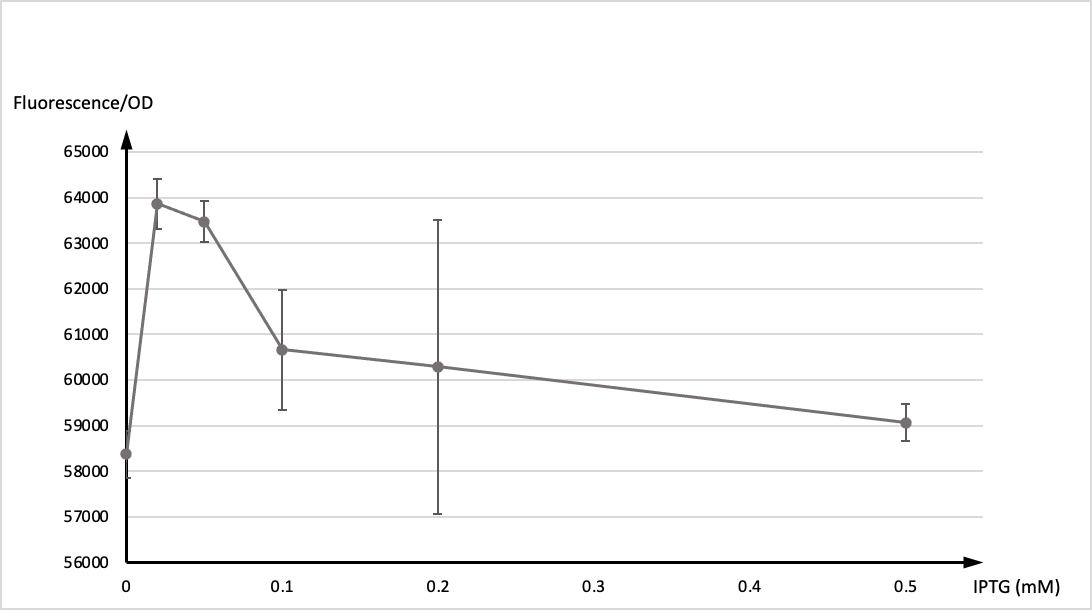
In the experiment, single colony was inoculated into LB medium, and cultivated vernight at 37° as seed culture. The seed culture was inoculated into LB medium as 1:100, and IPTG were added at 0h respectively. The concentration of IPTG was 0, 0.02, 0.05, 0.1, 0.2, 0.5mM, and two parallel samples for each concentration. 200 μl of each sample were taken to measure the fluorescence after 12 hours of induction.
Then, we tested OD values and the expression of GFP of two groups respectively. The graph below showed the ratio of fluorescence value/OD value of these two datas.
It is generally recognised that T7 promoter is not active in ordinary hosts, however,we found that it still maintain active functions at low concentration of IPTG (0.1mM) acordding to our results. That may be due to the potential endogenous promoter on pET28b and the gene expression caused by its high copy number. Please be cautious when using engineered strains to produce highly toxic protein expression plasmids.
Jiangnan-China 2022's contribution
I Overview
II Reference
lxs123456