Difference between revisions of "Part:BBa K4248000"
| Line 25: | Line 25: | ||
The results showed that the antimicrobial peptide proteins had significant antibacterial effects at double dilution concentrations. A single antimicrobial peptide protein can be seen to inhibit the growth of about 60% of bacteria in the range of 5 to 25 times dilution. A detailed analysis of the antibacterial effect of each antimicrobial peptide is given below. | The results showed that the antimicrobial peptide proteins had significant antibacterial effects at double dilution concentrations. A single antimicrobial peptide protein can be seen to inhibit the growth of about 60% of bacteria in the range of 5 to 25 times dilution. A detailed analysis of the antibacterial effect of each antimicrobial peptide is given below. | ||
| + | [[File:T--Shanghai city--BBa K4248000-figure3.png|500px|thumb|center|Figure 3. Test results of protein Hydramacin-1 inhibiting bacterial growth..]] | ||
| + | We confirmed the ability to use the Hydramacin-1 antimicrobial peptide to inhibit bacterial growth by first testing it against DH5α. As shown in the graph, the less diluted the antimicrobial peptide is, the more impact it has on bacterial growth. Although the average OD600 absorbance for the solution that contains 100μl of bacteria and 100μl of the Hydramacin-1 antimicrobial peptide that has been diluted 625, 125, and 25 times are relatively lower than the OD600 absorbance of the negative control group, the error bars of these variations overlap with each other. The overlap in their error bars hints that their OD600 absorbance is not significantly different from each other, meaning that when diluted 625, 125, and 25 times, the antimicrobial peptide plays an insignificant role in bacterial inhibition. However, there is a decrease in OD600 absorbance after applying the antimicrobial peptide that has been diluted 5 times. The drop in OD600 absorbance is further emphasized when we directly applied the antimicrobial peptide that has not been diluted to the DH5α bacteria. With the error bars of both of these variations not overlapping with those of the negative control group, the data shows the feasibility of using Hydramacin-1 antimicrobial peptide as a way to inhibit bacterial growth. While there is a drastic decrease in the OD600 absorbance where the Hydramacin–1 antimicrobial peptide that has not been diluted was applied, the graph shows that Kanamycin, the antibody that was used in the positive control group, is still a better bacterial inhibitor. Nevertheless, we can continue to concentrate the peptide solution, making its concentration higher than 0.212mg/ml. With a higher concentration, the Hydramacin-1 antimicrobial peptide may be a better bacterial inhibitor compared to Kanamycin or other antibodies. | ||
| − | |||
| − | |||
<!-- --> | <!-- --> | ||
Revision as of 07:05, 26 September 2022
Hydramacin-1
Hydramacin-1
Contribution
Hydramacin-1 is a novel antimicrobial protein recently discovered during investigations of the epithelial defense of the ancient metazoan Hydra. The amino acid sequence of hydramacin-1 shows no sequence homology to any known antimicrobial proteins. Determination of the solution structure revealed that hydramacin-1 possesses a disulfide bridge-stabilized alphabeta motif. This motif is the common scaffold of the knottin protein fold. The structurally closest relatives are the scorpion oxin-like superfamily. Within this superfamily hydramacin-1 establishes a new family of proteins that all share antimicrobial activity. Hydramacin-1 is potently active against Gram-positive and Gram-negative bacteria including multi-resistant human pathogenic strains. It leads to aggregation of bacteria as an initial step of its bactericidal mechanism.
Engineering Success
1. Construction of the antimicrobial peptide expression plasmids
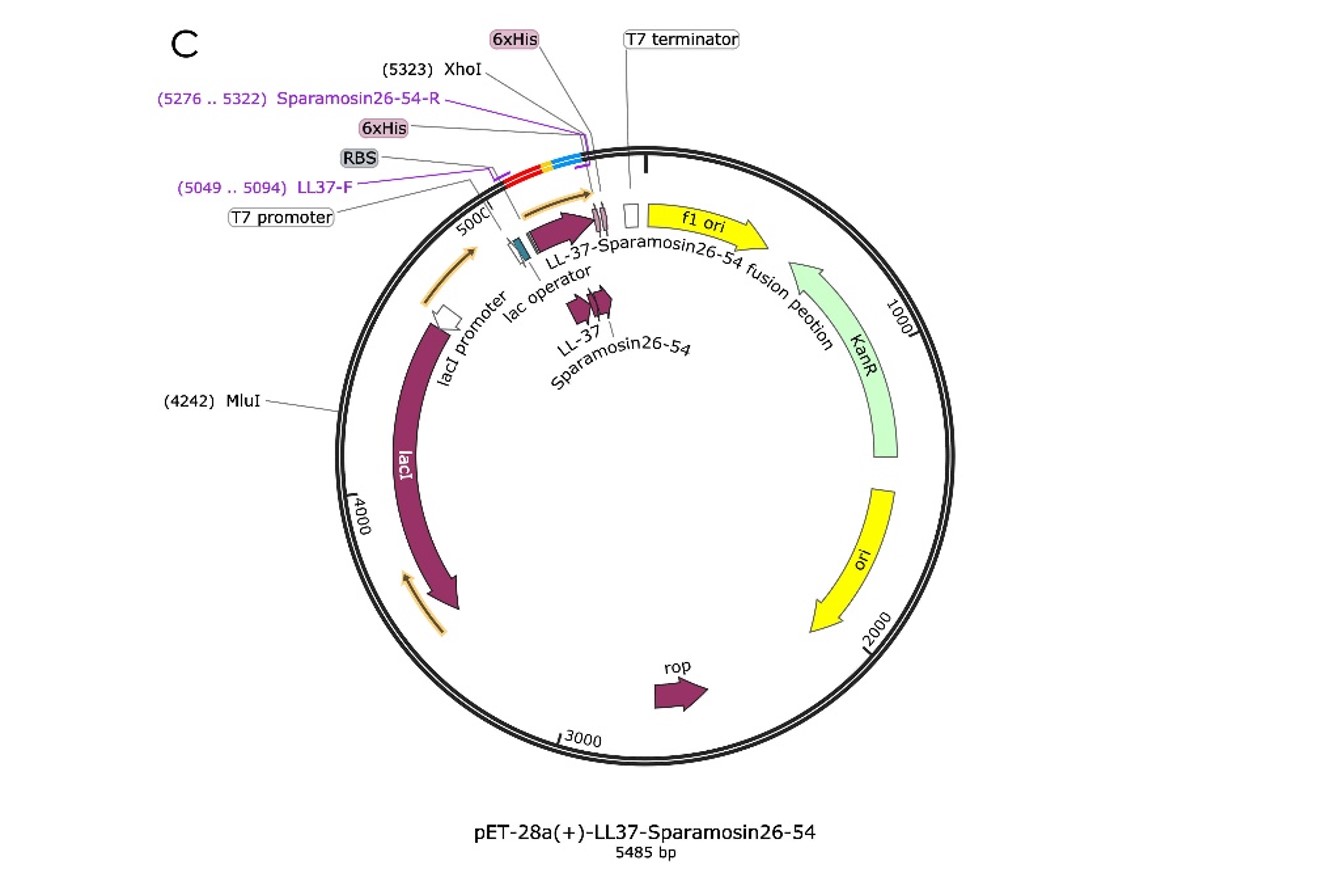
We design the plasmid: The DNA sequence of the four antimicrobial peptide Hydramacin-1, was inserted into the XbaI and XhoI sites of the pET-28a(+) vector. In order to build our plasmids, we let the synthetic company synthesize the target gene fragment, Hydramacin-1 integrate it into the pUC57 vector, Then, we amplify Hydramacin-1, by PCR (Figure1.), double-enzyme digestion, and ligase to pET28a(+) carrier.
We send the constructed recombinant plasmid to a sequencing company for sequencing. The returned sequencing comparison results showed that there were no mutations in the ORF region (Figure2.), and the plasmid was successfully constructed. So far, we have successfully obtained five recombinant plasmids, which were respectively on the pET28a(+) vector, which can be used to express antimicrobial peptide proteins.
2. Protein expression and purification
In order to obtain the five antimicrobial peptide proteins, we transferred the recombinant plasmids into E.coli BL21(DE3), expanded the culture in the LB medium, and added IPTG to induce protein expression when the OD600 reached 0.4. After overnight induction and culture, we collected the cells and ultrasonic fragmentation of cells to release the intracellular proteins. Next, we used nickel column purification to purify the antibacterial peptide protein we wanted. The concentration of each protein was measured as:0.212mg/mL Hydramacin-1.
At this point, we got the five antimicrobial peptide protein solutions we wanted.
3. Antibacterial ability test
Overview
To confirm the ability of the our purified antimicrobial peptide to inhibit bacterial growth, we used E.coli DH5-alpha as bacteria, and antibiotics as a positive control for bacteriostatic test experiments.
To better show the relationship between the concentration of antimicrobial peptides and the inhibition of bacterial growth, we added 100 μL of DH5α and 100 μL of different concentrations of the antimicrobial peptide to each of the five test tubes. Our five test tubes were filled with the antimicrobial peptide stock solution and diluted 1, 5, 25, 125, and 625 times solution, and repeated three times for each concentration to form the average data graph with error bars.
The results showed that the antimicrobial peptide proteins had significant antibacterial effects at double dilution concentrations. A single antimicrobial peptide protein can be seen to inhibit the growth of about 60% of bacteria in the range of 5 to 25 times dilution. A detailed analysis of the antibacterial effect of each antimicrobial peptide is given below.
We confirmed the ability to use the Hydramacin-1 antimicrobial peptide to inhibit bacterial growth by first testing it against DH5α. As shown in the graph, the less diluted the antimicrobial peptide is, the more impact it has on bacterial growth. Although the average OD600 absorbance for the solution that contains 100μl of bacteria and 100μl of the Hydramacin-1 antimicrobial peptide that has been diluted 625, 125, and 25 times are relatively lower than the OD600 absorbance of the negative control group, the error bars of these variations overlap with each other. The overlap in their error bars hints that their OD600 absorbance is not significantly different from each other, meaning that when diluted 625, 125, and 25 times, the antimicrobial peptide plays an insignificant role in bacterial inhibition. However, there is a decrease in OD600 absorbance after applying the antimicrobial peptide that has been diluted 5 times. The drop in OD600 absorbance is further emphasized when we directly applied the antimicrobial peptide that has not been diluted to the DH5α bacteria. With the error bars of both of these variations not overlapping with those of the negative control group, the data shows the feasibility of using Hydramacin-1 antimicrobial peptide as a way to inhibit bacterial growth. While there is a drastic decrease in the OD600 absorbance where the Hydramacin–1 antimicrobial peptide that has not been diluted was applied, the graph shows that Kanamycin, the antibody that was used in the positive control group, is still a better bacterial inhibitor. Nevertheless, we can continue to concentrate the peptide solution, making its concentration higher than 0.212mg/ml. With a higher concentration, the Hydramacin-1 antimicrobial peptide may be a better bacterial inhibitor compared to Kanamycin or other antibodies.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal AgeI site found at 118
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI.rc site found at 132