Difference between revisions of "Part:BBa K4096002"
| Line 30: | Line 30: | ||
1, 7 and 8 plasmids were sent to sequence. | 1, 7 and 8 plasmids were sent to sequence. | ||
| − | [[File:T--Shanghai Metro HS--BBa K4096004-Figure6.png|500px|thumb|center|Figure | + | [[File:T--Shanghai Metro HS--BBa K4096004-Figure6.png|500px|thumb|center|Figure 5 The result of sequencing for plasmid pET25b-PKC-OP..]] |
Sequencing feedback shows we have obtained the correct plasmids which is consistent with their DNA profiles. | Sequencing feedback shows we have obtained the correct plasmids which is consistent with their DNA profiles. | ||
== Proof of function == | == Proof of function == | ||
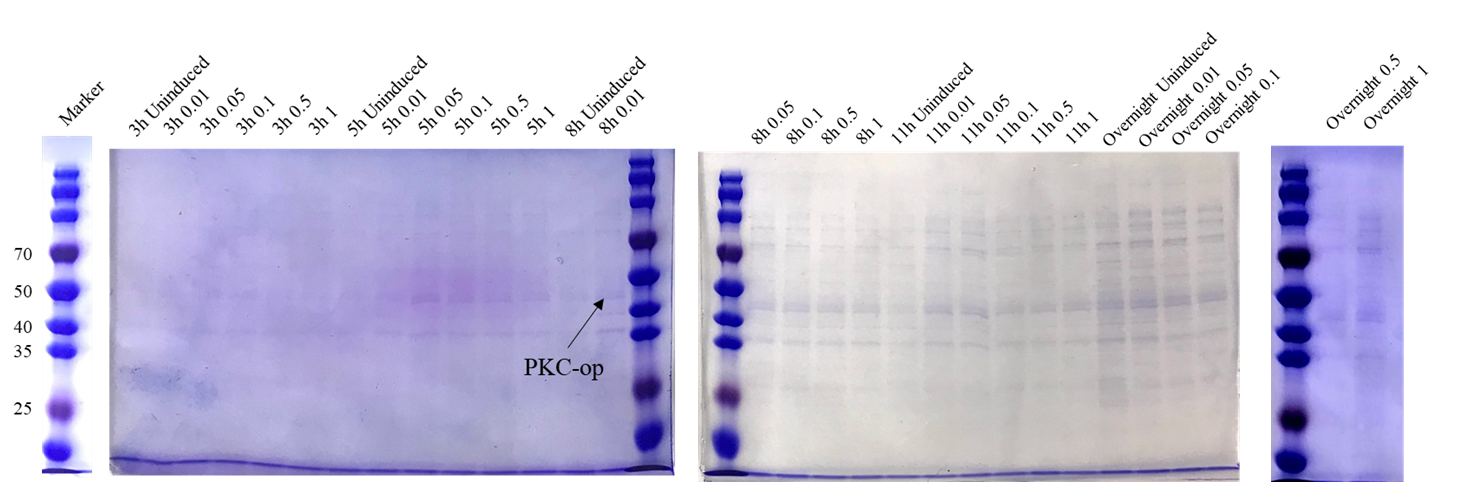
| − | Different induction conditions were tested for protein expression. As the pET-25b vector contains a pelB signal peptide, the engineered strain would secret the protein into the medium. Therefore, we collected the culture supernatant after induction and ran SDS-PAGE for verification (Figure | + | Different induction conditions were tested for protein expression. As the pET-25b vector contains a pelB signal peptide, the engineered strain would secret the protein into the medium. Therefore, we collected the culture supernatant after induction and ran SDS-PAGE for verification (Figure 6). |
| − | [[File:T--Shanghai Metro HS--BBa K4096002-Figure5.png|500px|thumb|center|Figure | + | [[File:T--Shanghai Metro HS--BBa K4096002-Figure5.png|500px|thumb|center|Figure 6. SDS-PAGE analysis of culture supernatant under different induction conditions..]] |
| − | The theoretical molecular weight of the PKC cellulase is 45.6 kDa. As seen from the SDS-PAGE (Figure | + | |
| + | The theoretical molecular weight of the PKC cellulase is 45.6 kDa. As seen from the SDS-PAGE (Figure 6), there is a wide band just above 40 kDa and it indicates that we have obtained the PKC cellulase. | ||
== References == | == References == | ||
Revision as of 02:10, 20 October 2021
Contents
- 1 BBa_K4096002
- 2 Usage and Biology
- 3 Construct design
- 4 Experimental approach
- 5 Proof of function
- 6 References
- 6.1 MUCK R,NADEAU E,MCALLISTER T,et al. Silage review: recent advances and future uses of silage additives [J]. J Dairy Sci,
- 6.2 刘海燕,张鹏举,王秀飞,等. 正交试验法优化玉米秸秆穰叶青贮发酵剂的研究 [J]. 中国饲料,2018 ( 11) : 74-79.
- 6.3 Coward-Kelly G., Aiello-Mazzari C., Kim S., Granda C., and Holtzapple M., 2003, Suggested improvements to the standard filter paper assay used to measure cellulase activity,Biotechnology & Bioengineering, 82(6): 745-749.
- 6.4 Ghose T.K., 1987, Measurement of cellulase activities, Pure & Appl Chem, 59(2): 257-268.
BBa_K4096002
Name: PKC-OP
Base Pairs: 1086bp
Origin: Pseudomonas aeruginosa, genome
Properties: A coding sequence of alkali cellulase.
Usage and Biology
BBa_K4096002 is a coding sequence of alkali cellulase (PKC-OP) from Pseudomonas aeruginosa. Cellulases break down the cellulose molecule into monosaccharides ("simple sugars") such as beta-glucose, or shorter polysaccharides and oligosaccharides. Several different kinds of cellulases are known, which differ structurally and mechanistically. Synonyms, derivatives, and specific enzymes associated with the name "cellulase" include endo-1,4-beta-D-glucanase (beta-1,4-glucanase, beta-1,4-endoglucan hydrolase, endoglucanase D, 1,4-(1,3,1,4)-beta-D-glucan 4-glucanohydrolase), carboxymethyl cellulase (CMCase), avicelase, celludextrinase, cellulase A, cellulosin AP, alkali cellulase, cellulase A 3, 9.5 cellulase, and pancellase SS.
Construct design
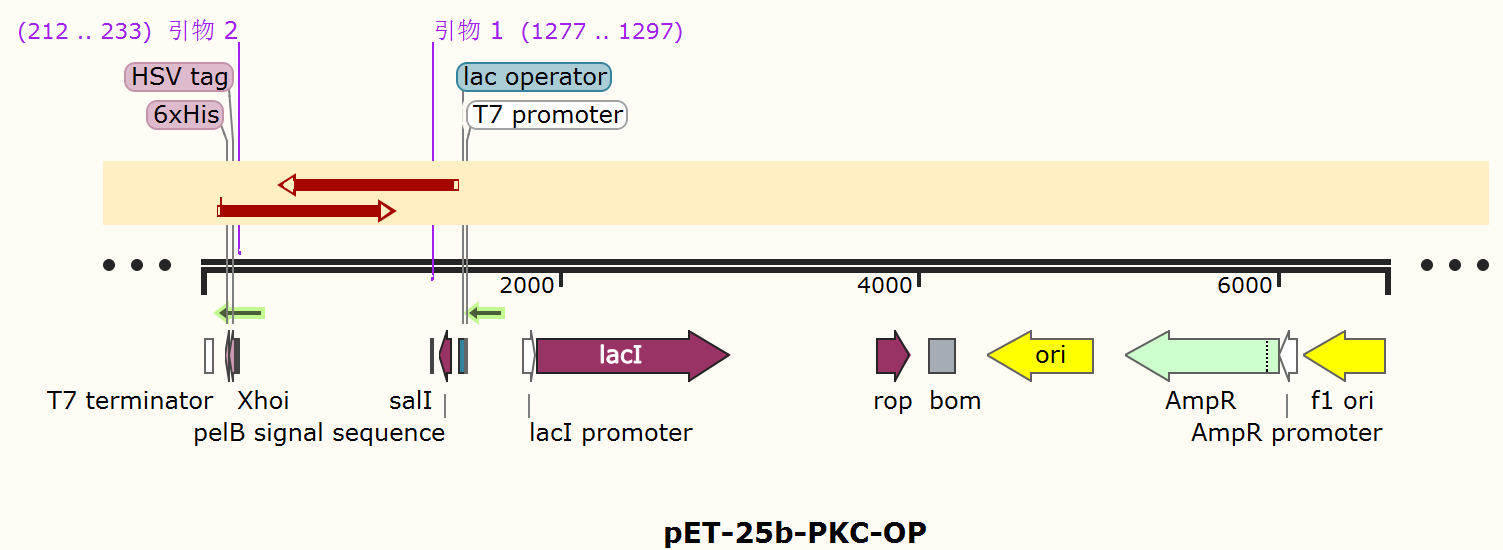
The alkaline cellulase gene from Pseudomonas aeruginosa PKC-001 was selected and we performed codon optimization on PKC-001 sequence to get PKC-OP, which was inserted into the pET-25b vector which contains a pelB signal peptide (Figure 1). The recombinant plasmid then was transformed it to E. coli BL21(DE3) strain to produce cellulase.
The profiles of every basic part are as follows:
Experimental approach
Construction of recombinant plasmid
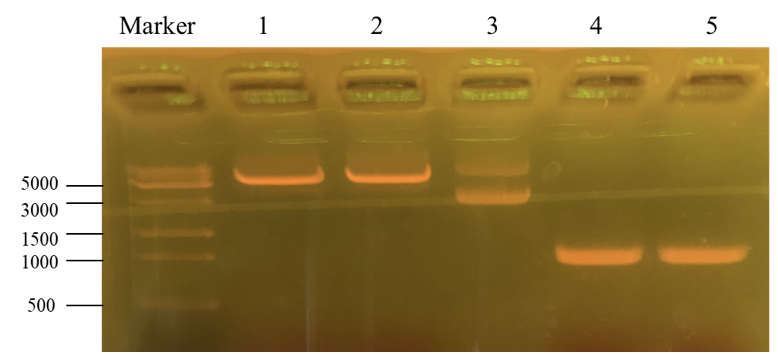
This picture is the nucleic acid electrophoresis result of enzyme cutting and PCR (By performing PCR, we can obtain the desirable PKC-OP’s gene segment.). Column “marker” is a column that is used to show the position of different lengths of genes. In this step, our aim is to verify whether these results are the desired ones. Number 1 and 2 is the result for pET-25b after enzyme digestion. Additionally, number 3 is the pET-25b without enzyme cutting. At last, number 4 and 5 is the PKC-OP after PCR. As our experiment moves on, the concentration of our PKC-OP and pET-25b are 233.2ng/μL and 17.8ng/μL. Lastly, we use the homologous combination to combine them.
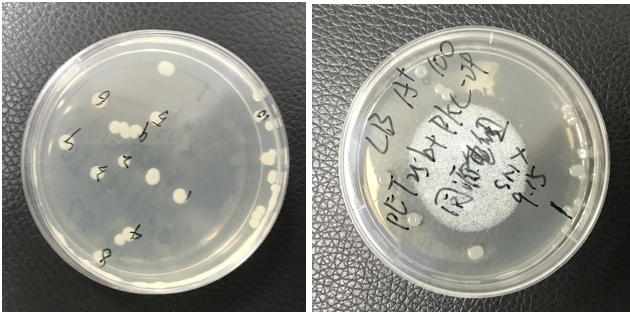
The pET25b-PKC-OP was constructed. The plate shows monoclonals of pET25b-PKC-OP constructs.
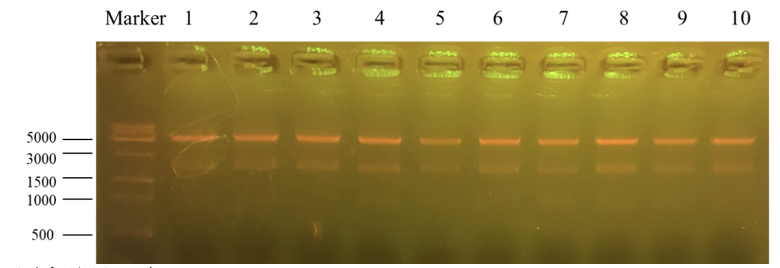
Column “marker” is a column that is used to show the position of different lengths of genes. Number 1 to 10 is the result for recombinant plasmid pET25b-PKC-OP after Apa1 enzyme digestion. We get two bands of 4712bp and 1894bp. It further indicates that the obtained monoclonals were positive monoclonals containing the recombinant plasmid. 1, 7 and 8 plasmids were sent to sequence.
Sequencing feedback shows we have obtained the correct plasmids which is consistent with their DNA profiles.
Proof of function
Different induction conditions were tested for protein expression. As the pET-25b vector contains a pelB signal peptide, the engineered strain would secret the protein into the medium. Therefore, we collected the culture supernatant after induction and ran SDS-PAGE for verification (Figure 6).
The theoretical molecular weight of the PKC cellulase is 45.6 kDa. As seen from the SDS-PAGE (Figure 6), there is a wide band just above 40 kDa and it indicates that we have obtained the PKC cellulase.