Difference between revisions of "Part:BBa K3846013"
Crisprcaspr (Talk | contribs) |
|||
| Line 3: | Line 3: | ||
<partinfo>BBa_K3846013 short</partinfo> | <partinfo>BBa_K3846013 short</partinfo> | ||
| − | + | This part contains the codon-optimised coding sequence of the Cytochrome P450 1A1 from <i>Rattus norvegicus</i>. It is a cytochrome P450 monooxygenase involved in the metabolism of various endogenous substrates, including fatty acids, steroid hormones and vitamins. Mechanistically, CYP1A1 uses molecular oxygen inserting one oxygen atom into a substrate, and reducing the second into a water molecule, with two electrons provided by NADPH via cytochrome P450 reductase (CPR; NADPH-ferrihemoprotein reductase). The enzyme catalyzes the hydroxylation of carbon-hydrogen bonds. For example, it exhibits high catalytic activity for the formation of hydroxyestrogens from estrone (E1) and 17beta-estradiol (E2), namely 2-hydroxy E1 and E2, as well as D-ring hydroxylated E1 and E2 at the C15alpha and C16alpha positions. It is often used as model cytochrome P450 enzyme because the conversion of 7-ethoxycoumarin to 7-methoxycoumarin can be directly measured by changes in fluorescence. | |
| − | + | The corresponding phytobrick [https://parts.igem.org/Part:BBa_K3846013 BBa_K3846101] was used to create different constructs to test the effect of linker sequences in Cytochrome P450 reductase fusion proteins. | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
<!-- Add more about the biology of this part here | <!-- Add more about the biology of this part here | ||
| Line 27: | Line 19: | ||
<partinfo>BBa_K3846013 parameters</partinfo> | <partinfo>BBa_K3846013 parameters</partinfo> | ||
<!-- --> | <!-- --> | ||
| + | |||
| + | |||
| + | |||
| + | ===iGEM Hamburg 2021 part collection=== | ||
| + | Terpenoids are an important group of natural products used as biofuels, drugs or fragrances. Naturally occuring in plants it has been shown that microbial terpene production in microorganisms like yeast, E. coli or cyanobacteria is possible. | ||
| + | Nevertheless iGEM projects seem to rarely focus on this interesting class of natural products which is correlated with a lack of useful parts inside the iGEM registry. | ||
| + | |||
| + | Fortunately we were able to change that and designed a novel golden gate based toolbox which allows. | ||
| + | <ol style="list-style-type:lower-alpha"> | ||
| + | <li>production of terpenoid precursors and simple terpenoids</li> | ||
| + | <li>creation of CYP P450-reductase fusion enzymes to optimise processing of terpenoid precursors and production of bioactive target products</li> | ||
| + | <li>modularity of the system to enable exchange of linker sequences/promoters/etc. (MoClo-compatible toolbox)</li> | ||
| + | </ol> | ||
| + | |||
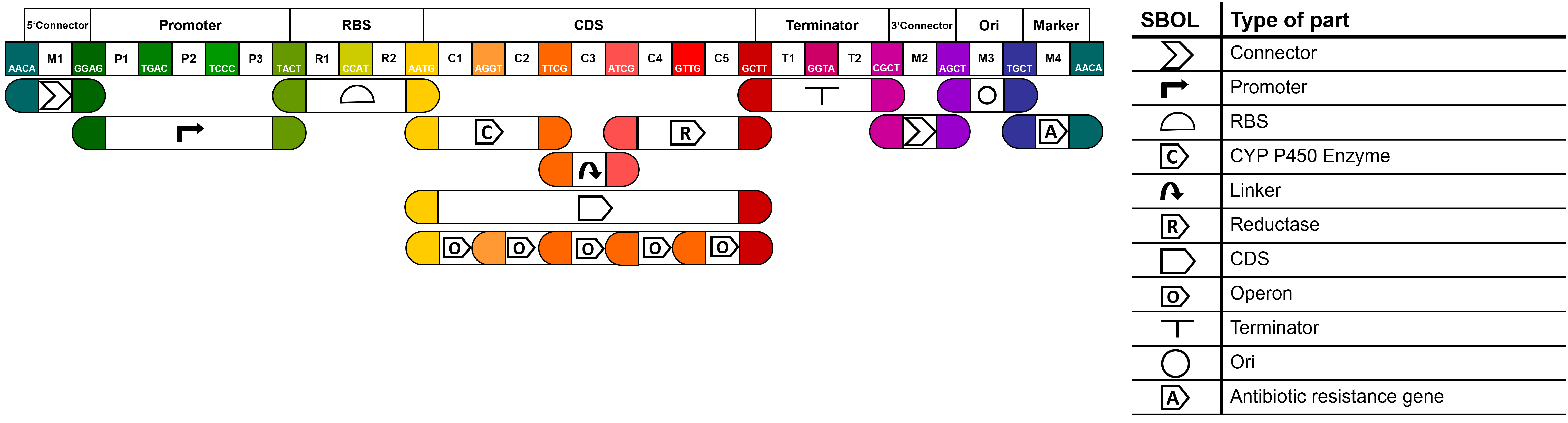
| + | ===MoClo-based Part Design 2.0=== | ||
| + | <p>To improve the usefulness of our parts, we then aimed to make our parts compatible with the MoClo standard of goten gate based IIS restriction enzyme assembly. Thereby we expanded the Common Genetic Syntax for fusion sites to allow the creation of a) fusion proteins connected by linker sequences and b) multiple CDS expressed in an operon. | ||
| + | More useful information and an overview of all our parts can be found on our [https://2021.igem.org/Team:Hamburg/Part_Collection wiki]. | ||
| + | |||
| + | [[File:T--Hamburg--parts overview MolClo.png|600px|thumb|left|'''Figure 1''': <b> MoClo syntax of the part collection. </b> <br>]] | ||
Latest revision as of 15:48, 19 October 2021
Cytochrome P450 1A1
This part contains the codon-optimised coding sequence of the Cytochrome P450 1A1 from Rattus norvegicus. It is a cytochrome P450 monooxygenase involved in the metabolism of various endogenous substrates, including fatty acids, steroid hormones and vitamins. Mechanistically, CYP1A1 uses molecular oxygen inserting one oxygen atom into a substrate, and reducing the second into a water molecule, with two electrons provided by NADPH via cytochrome P450 reductase (CPR; NADPH-ferrihemoprotein reductase). The enzyme catalyzes the hydroxylation of carbon-hydrogen bonds. For example, it exhibits high catalytic activity for the formation of hydroxyestrogens from estrone (E1) and 17beta-estradiol (E2), namely 2-hydroxy E1 and E2, as well as D-ring hydroxylated E1 and E2 at the C15alpha and C16alpha positions. It is often used as model cytochrome P450 enzyme because the conversion of 7-ethoxycoumarin to 7-methoxycoumarin can be directly measured by changes in fluorescence.
The corresponding phytobrick BBa_K3846101 was used to create different constructs to test the effect of linker sequences in Cytochrome P450 reductase fusion proteins.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
iGEM Hamburg 2021 part collection
Terpenoids are an important group of natural products used as biofuels, drugs or fragrances. Naturally occuring in plants it has been shown that microbial terpene production in microorganisms like yeast, E. coli or cyanobacteria is possible. Nevertheless iGEM projects seem to rarely focus on this interesting class of natural products which is correlated with a lack of useful parts inside the iGEM registry.
Fortunately we were able to change that and designed a novel golden gate based toolbox which allows.
- production of terpenoid precursors and simple terpenoids
- creation of CYP P450-reductase fusion enzymes to optimise processing of terpenoid precursors and production of bioactive target products
- modularity of the system to enable exchange of linker sequences/promoters/etc. (MoClo-compatible toolbox)
MoClo-based Part Design 2.0
To improve the usefulness of our parts, we then aimed to make our parts compatible with the MoClo standard of goten gate based IIS restriction enzyme assembly. Thereby we expanded the Common Genetic Syntax for fusion sites to allow the creation of a) fusion proteins connected by linker sequences and b) multiple CDS expressed in an operon. More useful information and an overview of all our parts can be found on our wiki.