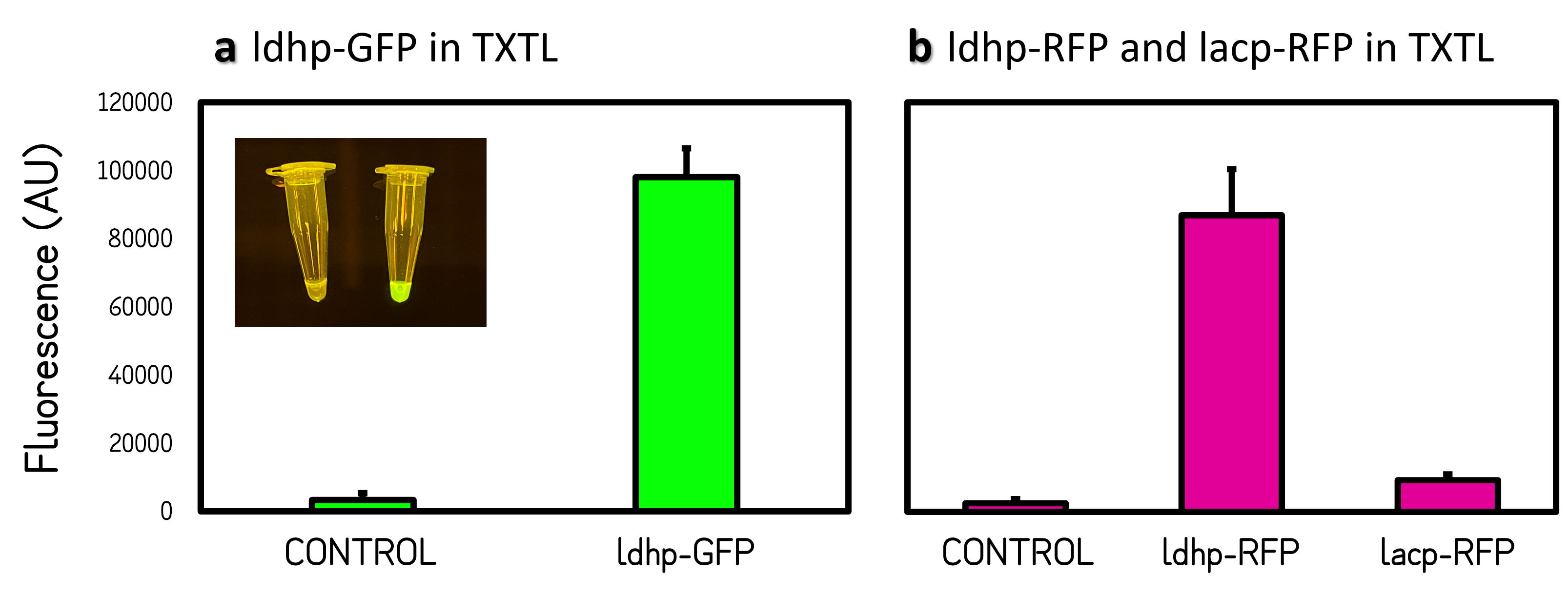Difference between revisions of "Part:BBa K3728008"
| Line 59: | Line 59: | ||
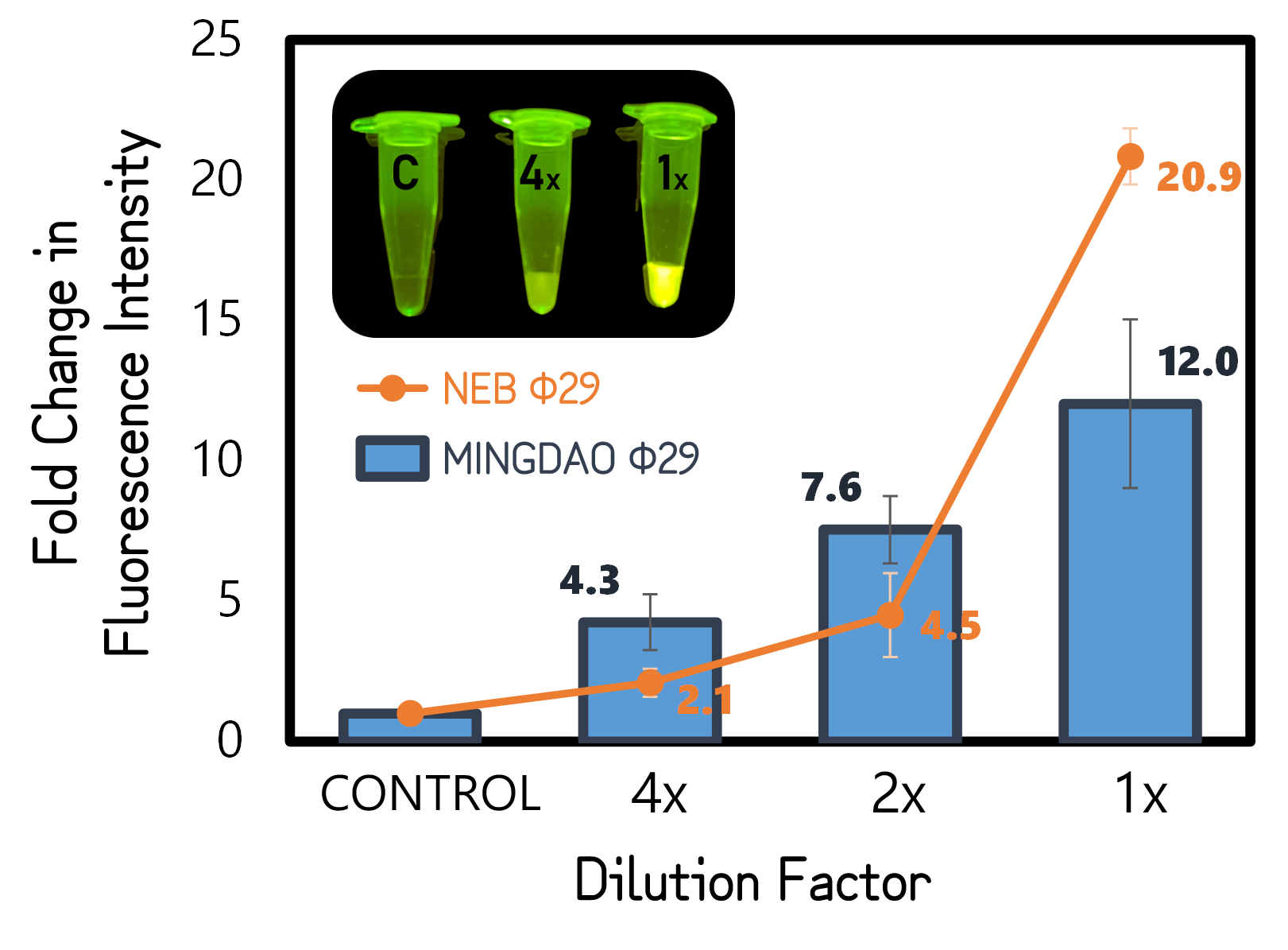
<span style="color:#00000000">This</span>We performed RCA by mixing a circular ssDNA and primer in the buffer with our purified phi29 DNA polymerase (Φ29) from TXTL using Salmonella extracts or a commercial recombinant Φ29 from New England Biolabs Inc. (NEB) as a positive control. The Φ29 enzymes were diluted with various factors in the assay. After incubation at 30°C for 1 hour, the RCA products were stained with EvaGreen DNA dye and subjected to a microplate reader to measure signals at Ex/Em=500/530 nm. And the fold changes in fluorescence intensity were calculated by dividing the values from Φ29-untreated groups (as controls). As shown in Fig. 11, a 12-fold change was achieved with our Φ29, indicating the functionality and the activity of ldhp-Phi29 DNA pol-Tr/pTol2 that are comparable to the commercial NEB Φ29 enzymes. Moreover, the green fluorescence can readily be seen with a blue led light, even using a 4 times diluted Φ29, proving the super high processivity of phi29 DNA polymerase in DNA amplification. | <span style="color:#00000000">This</span>We performed RCA by mixing a circular ssDNA and primer in the buffer with our purified phi29 DNA polymerase (Φ29) from TXTL using Salmonella extracts or a commercial recombinant Φ29 from New England Biolabs Inc. (NEB) as a positive control. The Φ29 enzymes were diluted with various factors in the assay. After incubation at 30°C for 1 hour, the RCA products were stained with EvaGreen DNA dye and subjected to a microplate reader to measure signals at Ex/Em=500/530 nm. And the fold changes in fluorescence intensity were calculated by dividing the values from Φ29-untreated groups (as controls). As shown in Fig. 11, a 12-fold change was achieved with our Φ29, indicating the functionality and the activity of ldhp-Phi29 DNA pol-Tr/pTol2 that are comparable to the commercial NEB Φ29 enzymes. Moreover, the green fluorescence can readily be seen with a blue led light, even using a 4 times diluted Φ29, proving the super high processivity of phi29 DNA polymerase in DNA amplification. | ||
| − | [[File:T--Mingdao--008photo6 1019.png| | + | [[File:T--Mingdao--008photo6 1019.png|450px|left]] |
<br><br><br><br><br><br>Figure 5 | RCA assay using TXTL-expressed (MINGDAO) or commercial (NEB) phi29 DNA polymerase (Φ29). The commercial Φ29 was purchased from NEB with a defined activity by units. The control was set without Φ29 treatment. The concentration and dilutions of enzymes were, respectively, 4, 2, 1 g/l for MINGDAO Φ29 and 1, 0.5, 0.25 units for NEB Φ29. The EvaGreen DNA binding signals were read at Ex/Em=500/530 nm in BioTek Synergy H1 Microplate Reader. | <br><br><br><br><br><br>Figure 5 | RCA assay using TXTL-expressed (MINGDAO) or commercial (NEB) phi29 DNA polymerase (Φ29). The commercial Φ29 was purchased from NEB with a defined activity by units. The control was set without Φ29 treatment. The concentration and dilutions of enzymes were, respectively, 4, 2, 1 g/l for MINGDAO Φ29 and 1, 0.5, 0.25 units for NEB Φ29. The EvaGreen DNA binding signals were read at Ex/Em=500/530 nm in BioTek Synergy H1 Microplate Reader. | ||
Revision as of 06:46, 19 October 2021
ldhp-Phi29 DNA pol-Tr/pTol2
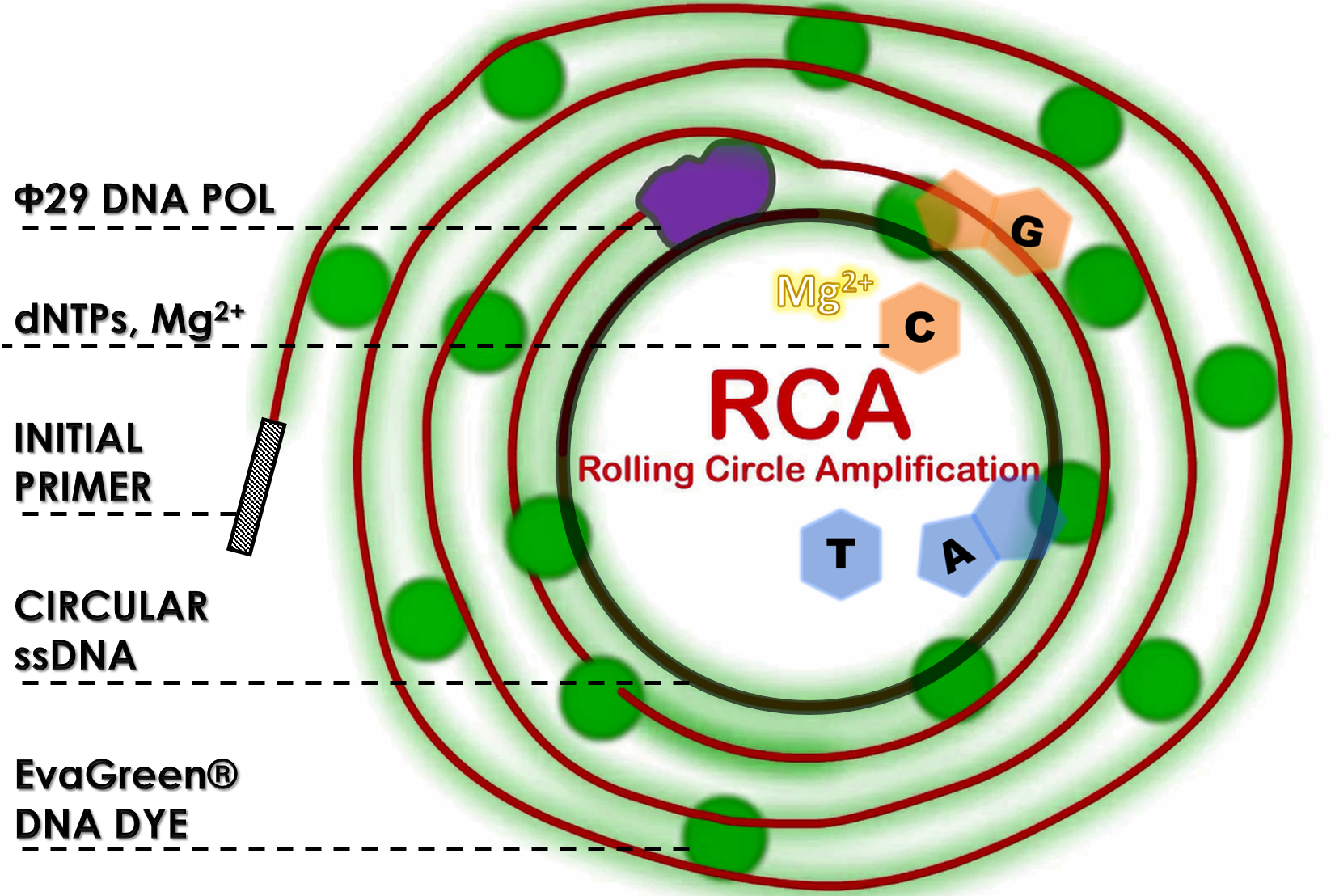
ThisPhi29 (Φ29) DNA polymerase is an exceptional processive polymerase possessing highly isothermal amplification and strong strand displacement activities[1][2]. The polymerase has been increasingly used in rolling circle amplification (RCA) assay that make long ssDNA strands using an initial primer and a single-stranded circular DNA template in the buffer containing dNTPs and Mg2+. The large amounts of amplified DNAs can be quickly generated within 1 hour and easily be measured with fluorescent DNA binding dye (e.g., EvaGreen® Dye).
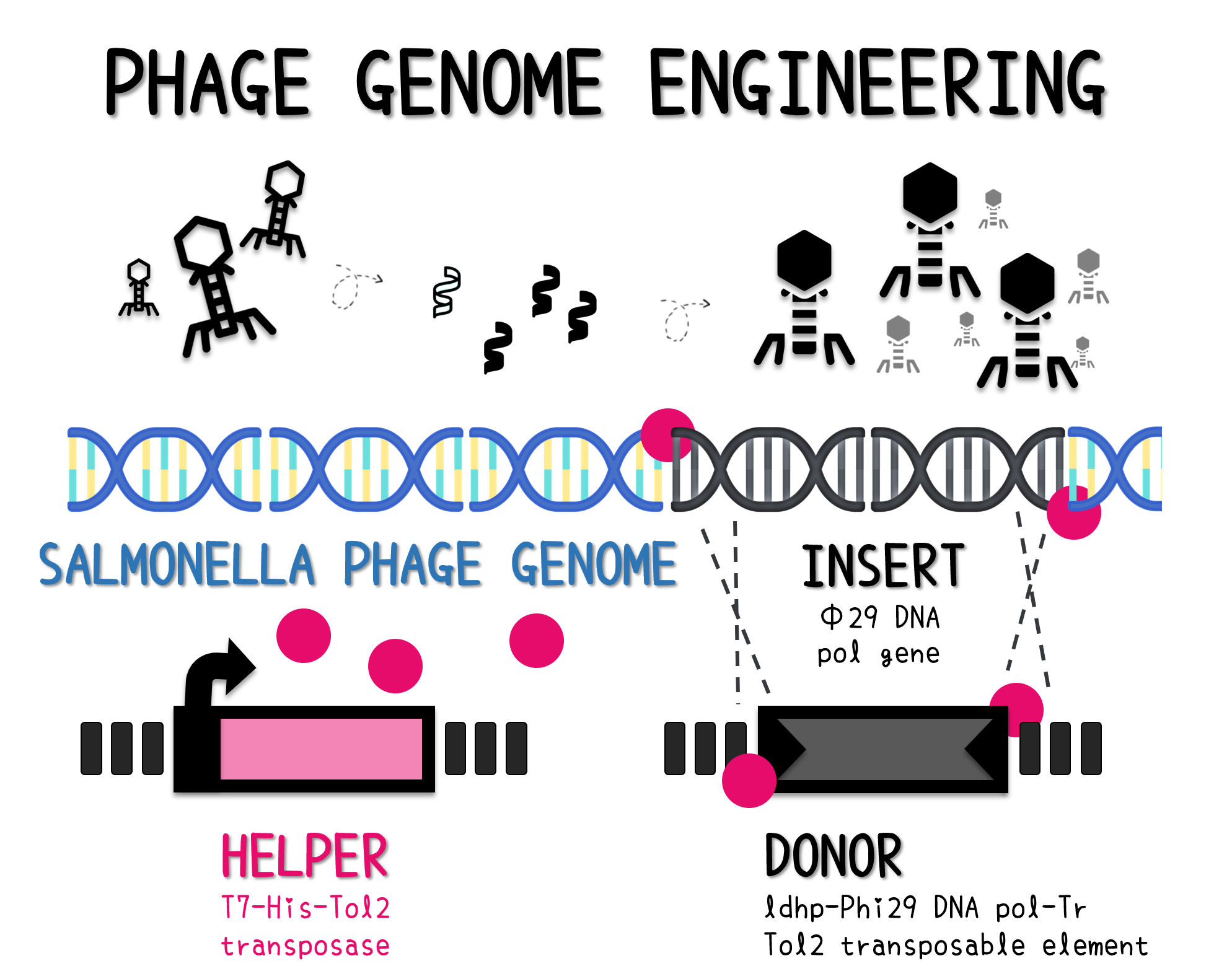
ThisTo engineer an isolated Salmonella phage genome with unknown full genome sequence, we want to use in vitro Tol2 transposon system with Tol2 transposase and Tol2 transposable element to make a recombinant phage genome carrying phi29 DNA polymerase gene.
ThisTo achieve that, we built up a Tol2 transposable vector of the BioBrick part of ldhp-Phi29 DNA pol-Tr/pTol2 consisting a constitutive promoter of ldhp and a His-tagged phi29 DNA polymerase gene with a double terminator. We verified the functionality of each basic part on the ldhp-Phi29 DNA pol-Tr/pTol2 in the following. Finally, we demonstrated a Salmonella phage engineered with this vector can be a useful Salmonella detection tool.
CONSTRUCTION
Tol2 Transposable Element
ThisMinimal Tol2 transposable element (mTol2) has been characterized that is composed of 200-bp left arm and 150-bp right arm[3]. The 19-bp to 11-kbp DNA inserts between the arms can be excised and transposed efficiently by Tol2 transposase (TPase). Therefore, we’d like to make a BioBrick compatible vector based on Tol2 mobile element (pTol2), which can be further assembled through a BioBrick standard EcoRI-XbaI-SpeI-PstI rule.
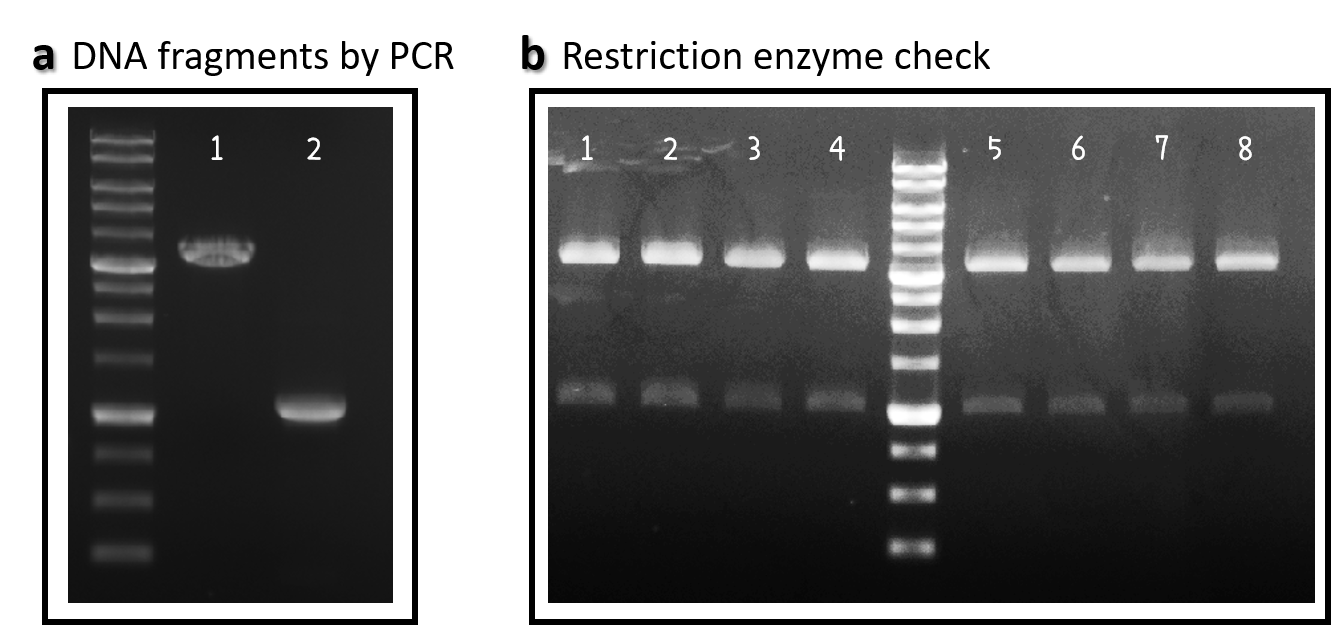
ThisWe obtained the backbone of pBSII-SK-mTol2-MCS from Addgene (Plasmid #51817), which was given by Elly Tanaka[4]. We deleted restriction enzyme sites in MCS and generated novel BioBrick Prefix (EcoRI-NotI-XbaI) and BioBrick Suffix (SpeI-NotI-PstI) elements in the both ends by PCR. The resulting DNA plasmid backbone called pTol2 (Part:BBa_K3376002) was further assembled with the Part BBa_J04450 (i.e., the iGEM official standard insert on pSB1C3). The resulting J04450/pTol2 (Part:BBa_K3376003) was checked by PCR (Fig. 1a) and restriction enzymes (Fig. 1b) and also confirmed by sequencing.
Figure 1 | pTol2 and J04450/pTol2 constructs check. DNAs were run electrophoresis on 1% agarose gel with 1kb marker. (a) PCR producs of pTol2 (lane 1, 3429 bp) and BBa_J04450 (lane 2, 1112 bp). (b) 4 clones of J04450/pTol2 were checked by restriction enzymes (~ 3432 bp and ~1110 bp). Lanes 1-4 by EcoRI and SpeI. Lanes 5-8 by XbaI and PstI.
Ф29 DNA Polymerase
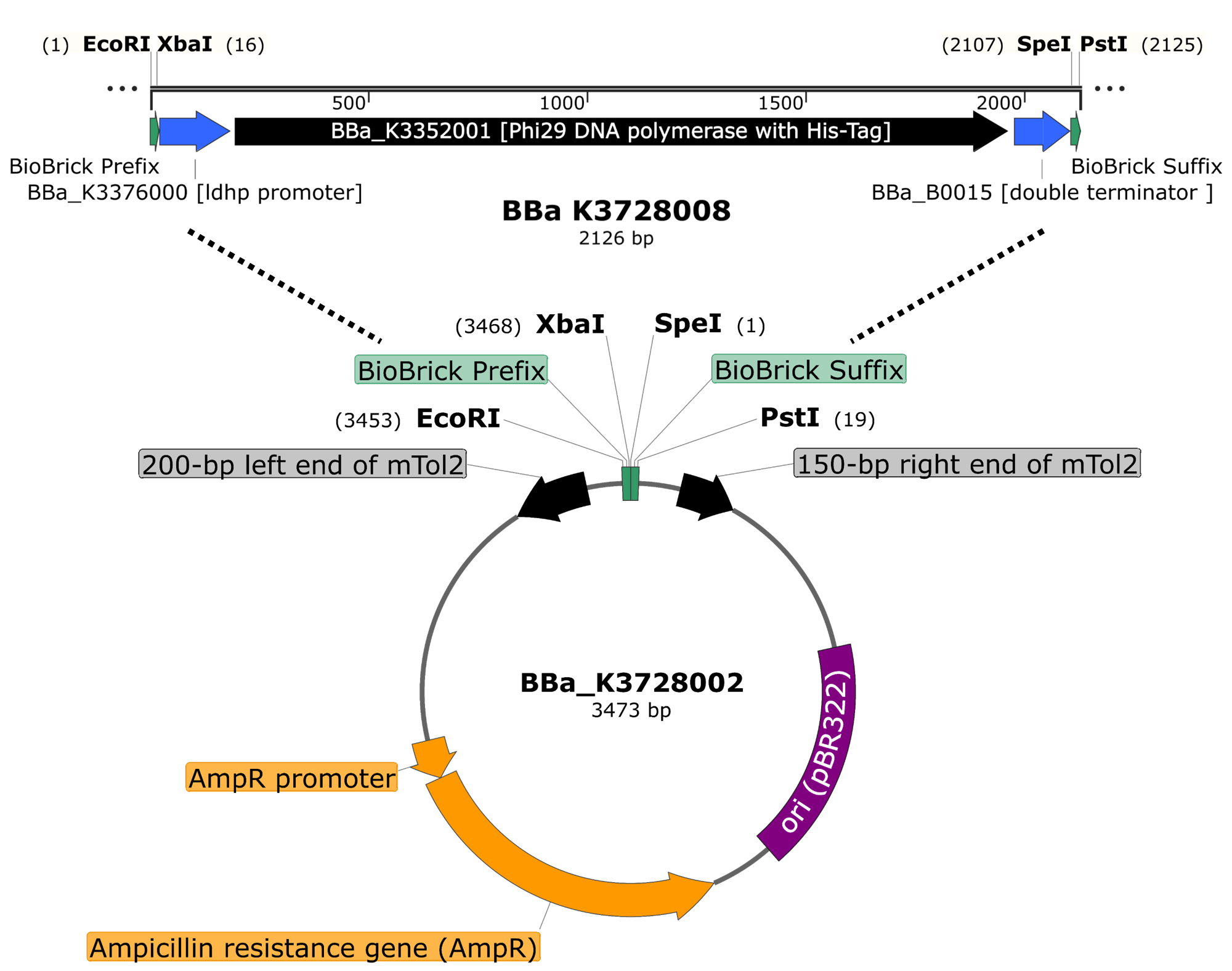
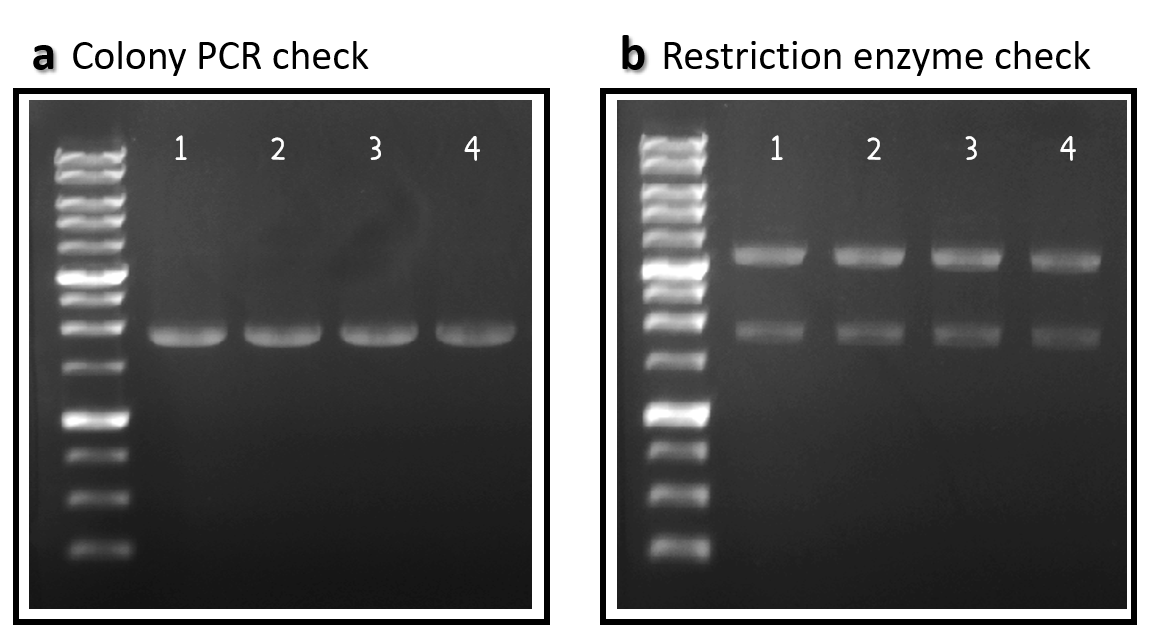
ThisTo engineer RCA reporter Salmonella phage, we requested Part:BBa_K3352001 (Φ29 DNA Polymerase with His-Tag and GS linker Sequence) from iGEM team TAS_Taipei in 2020. The part was assembled with ldhp promoter Part:BBa_K3376000 and a double terminator Part:BBa_B0015 onto the Tol2 mobile element vector (pTol2, Part:BBa_K3728002). The resulting composite part named ldhp-Phi29 DNA pol-Tr/pTol2 (Part:BBa_K3728008) was checked by colony PCR, restriction enzymes and sequencing (Fig. 2).
Figure 2 | ldhp-Phi29 DNA pol-Tr/pTol2 construct check. DNAs were run electrophoresis on 1% agarose gel with 1kb marker. (a) 4 colonies were subject to PCR with ldhp forward primer and a reverse primer in the end of Phi29 gene (PCR product size: 1965 bp). (b) The DNAs were extracted and digested by EcoRI and PstI (3573 and 1973 bps).
CELL-FREE TXTL SYSTEM
ThisIn vitro transcription and translation (TXTL) is a convenient cell-free system that has increasingly been developed to apply in synthetic biology[5][6]. In addition to achieve biosafety level, TXTL becomes powerful in prototype characterization of genetic parts, devices and circuits. Moreover, TXTL is particularly useful to express and purify proteins which are toxic, insoluble or unstable in cell-based system. Furthermore and amazingly, Dr. Vincent Noireau’s lab has demonstrated cell-free TXTL application in infectious bacteriophage production, in which T7 phage (40kbp, 77 genes, dsDNA) and T4 phage (170kbp, 289 genes, dsDNA) genome replication, synthesis, assembly can be performed in vitro just in a single test tube[7][8]
Promoter Activity
ThisTo test the TPase activity and Tol2 transposon system, we inserted a kanamycin resistance gene (KanR) cassette (Part:BBa_K3376004) and the reporters of ldhp-GFP-Tr(Part:BBa_K3376005), ldhp-RFP-Tr(Part:BBa_K3376006) and ldhp-amilCP-Tr(Part:BBa_K3376007) between the transposable elements on the pTol2 vector. ldhp is a constitutive and broad-host-range promoter, which was originally cloned and driving the lactate dehydrogenase gene in S. mutans. We have characterized the ldhp activities in S. mutans and E. coli in our project of iGEM 2020, as well as Salmonella and TXTL in this project of iGEM 2021.(Part:BBa_K3376000)
ThisThe GFP and RFP fluorescence intensities driven by ldhp on pTol2 vectors were measured at high level in TXTL reaction (Fig. 3). The strong GFP fluorescence can even be visualized by naked eyes under a Blue LED Illuminator. Compared the activities of ldhp to lac promoter (lacp), lacp is inhibited in TXTL because the extracts of E. coli Rosetta 2 (DE3) contains LacI repressor, which can be relieved by IPTG induction or using E. coli DH5α as extracts.
Figure 3 | Promoter activities on pTol2 vector in TXTL. GFP fluorescence was measured at Ex/Em = 500/530 nm using a microplate reader of BioTek Synergy H1. RFP was at Ex/Em = 586/611 nm. KanR/pTol2 in TXTX was set as a background control. AU means arbitrary unit. (a) ldhp-GFP-Tr/pTol2 activity in TXTL. The inset photo was captured under a blue LED light. (b) ldhp-RFP-Tr/pTol2 and J04450/pTol2 (i.e., lacp-RFP-Tr) in TXTL.
Protein Expression
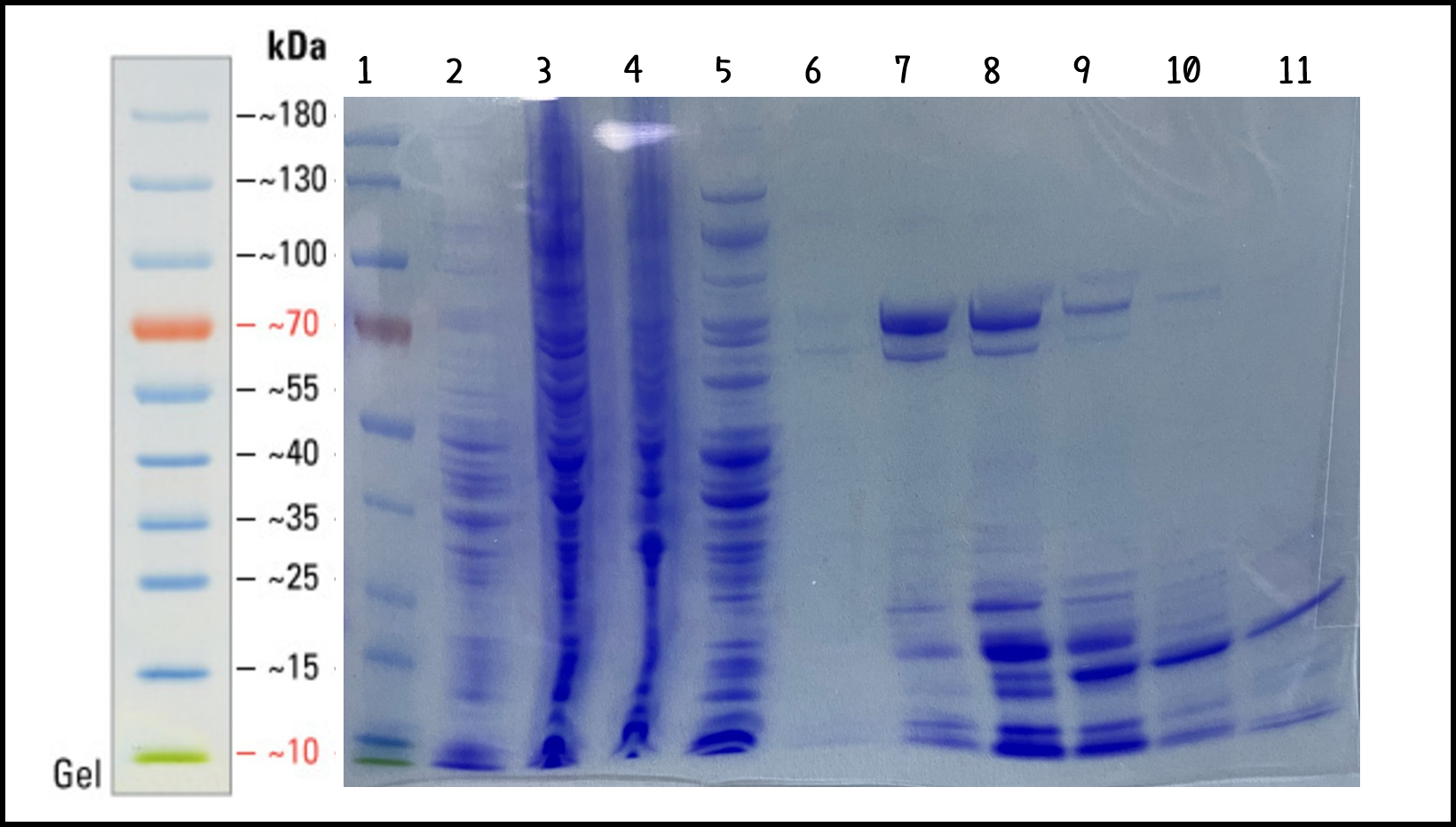
ThisTo characterize the function of recombinant phi29 DNA polymerase, TXTL cell-free system can solve the problem caused by the difficulty in bacterial transformation or without suitable plasmid vectors. We took the DNA of ldhp-Phi29 DNA pol-Tr/pTol2(Part:BBa K3728008)mixed into TXTL reaction with Salmonella extracts. The recombinant His-tagged phi29 DNA polymerase protein was purified through Nickel column and analyzed by SDS-PAGE and Coomassie Blue Staining (Fig. 4). The isolated proteins in Elution #4 and #5 were at the size of around 70 kDa as predicted (His-phi29 DNA polymerase: 590 amino acids, 68 kDa) and collected for the following studies.
Figure 4 | His-phi29 DNA polymerase was expressed in TXTL using Salmonella extracts and purified by Nickel column. 5 μg of protein lysates were analyzed by SDS-PAGE and Coomassie Blue Staining using 4–12% gradient gel (NuPAGE™, Thermo Fisher Scientific Inc.) Lane: (1) PageRuler™ Prestained Protein Ladder, (2) Salmonella cell extracts (no DNA control), (3) total lysates in TXTL, (4) flow-through, (5) wash-through, (6) Elution #3, (7) Elution #4, (8) Elution #5, (9) Elution #6, (10) Elution #7, (11) Elution #8.
RCA TESTING
ThisWe performed RCA by mixing a circular ssDNA and primer in the buffer with our purified phi29 DNA polymerase (Φ29) from TXTL using Salmonella extracts or a commercial recombinant Φ29 from New England Biolabs Inc. (NEB) as a positive control. The Φ29 enzymes were diluted with various factors in the assay. After incubation at 30°C for 1 hour, the RCA products were stained with EvaGreen DNA dye and subjected to a microplate reader to measure signals at Ex/Em=500/530 nm. And the fold changes in fluorescence intensity were calculated by dividing the values from Φ29-untreated groups (as controls). As shown in Fig. 11, a 12-fold change was achieved with our Φ29, indicating the functionality and the activity of ldhp-Phi29 DNA pol-Tr/pTol2 that are comparable to the commercial NEB Φ29 enzymes. Moreover, the green fluorescence can readily be seen with a blue led light, even using a 4 times diluted Φ29, proving the super high processivity of phi29 DNA polymerase in DNA amplification.
Figure 5 | RCA assay using TXTL-expressed (MINGDAO) or commercial (NEB) phi29 DNA polymerase (Φ29). The commercial Φ29 was purchased from NEB with a defined activity by units. The control was set without Φ29 treatment. The concentration and dilutions of enzymes were, respectively, 4, 2, 1 g/l for MINGDAO Φ29 and 1, 0.5, 0.25 units for NEB Φ29. The EvaGreen DNA binding signals were read at Ex/Em=500/530 nm in BioTek Synergy H1 Microplate Reader.
SALMONELLA PHAGE ENGINEERING & APPLICATION
Engineering through in vitro Tol2 Transposon system
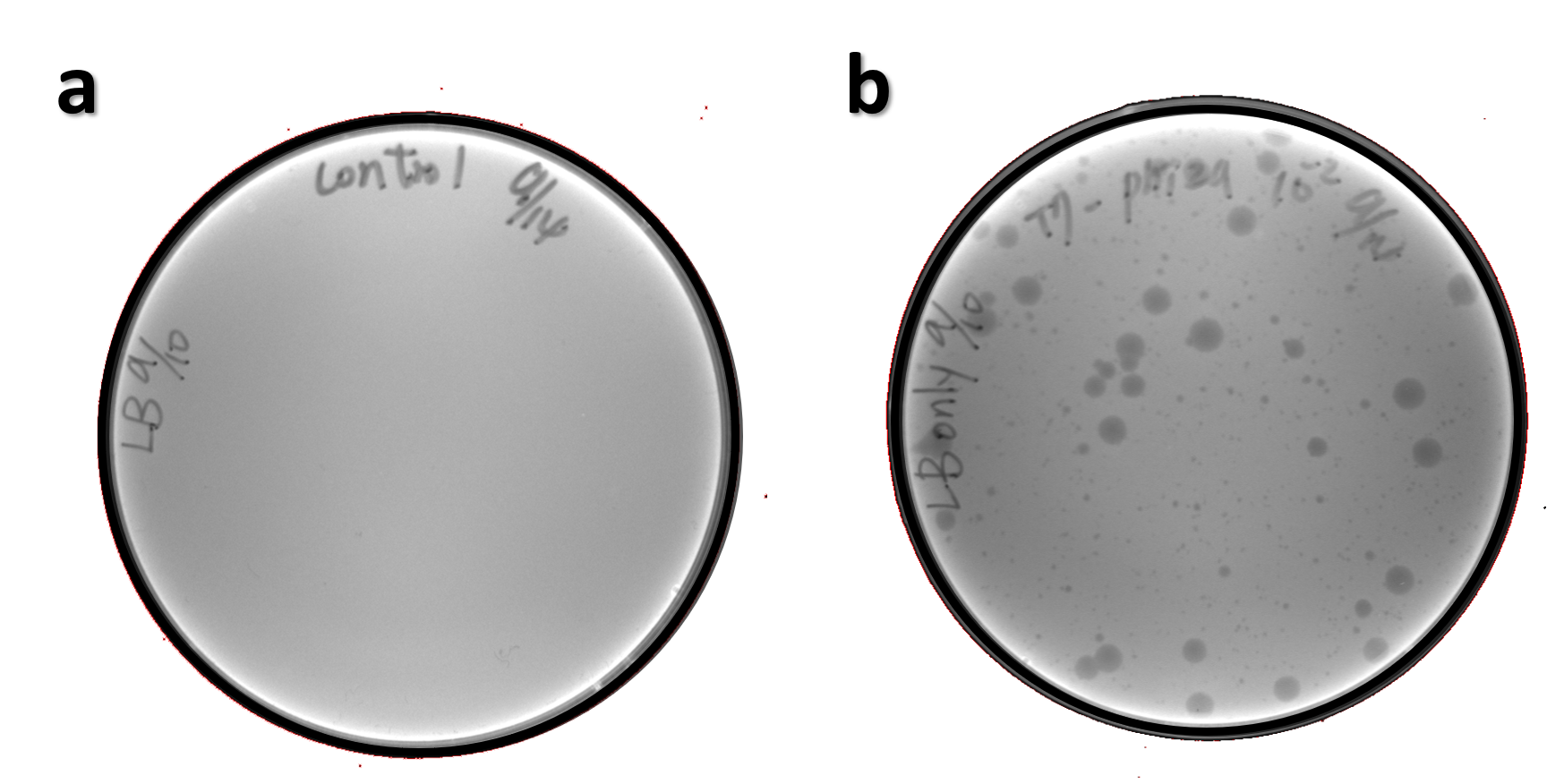
ThisThe genome of Salmonella phage #ST1 was extracted and engineered to carry phi29 DNA polymerase gene through the Tol2 transposon system. T7-His-Tol2 transposase was expressed in TXTL using IPTG-induced E. coli Rosetta 2(DE3) extracts, followed by purification with Nickel column. The DNA fragment of Tol2 transposable element carrying ldhp-Phi29 DNA polymerase-Tr (Ф29/Tol2) was amplified by PCR. The extracted Salmonella phage genome and the Ф29/Tol2 DNA fragment were incubated with Tol2 transposase at 30°C for 2 hours. The recombinant phage genome was subjected to TXTL based on the work of Jonghyeon Shin[7], where T7 phage genome can be replicated, synthesized, and assembled in a single cell-free reaction. The infectious phage of Salmonella phage #ST1::Ф29 made by TXTL using Salmonella extracts was tested on plaque assay with a culture of Salmonella on the LB agar plate. As shown in Fig. 5b, visible different sizes of plaques were formed on the agar plate, demonstrating infectious phages were produced in our TXTL system. As a control, the phage gDNA without TXTL reaction displayed no plaques (Fig. 5a), indicating the live phages from TXTL are not from the contaminated DNA in the process of genomic DNA extraction.
Figure 5 | The recombinant phage synthesis in TXTL. Plaques were formed on a lawn of Salmonella culture on the LB agar plate from TXTL reaction (b) compared to no plaques without TXTL reaction (a).
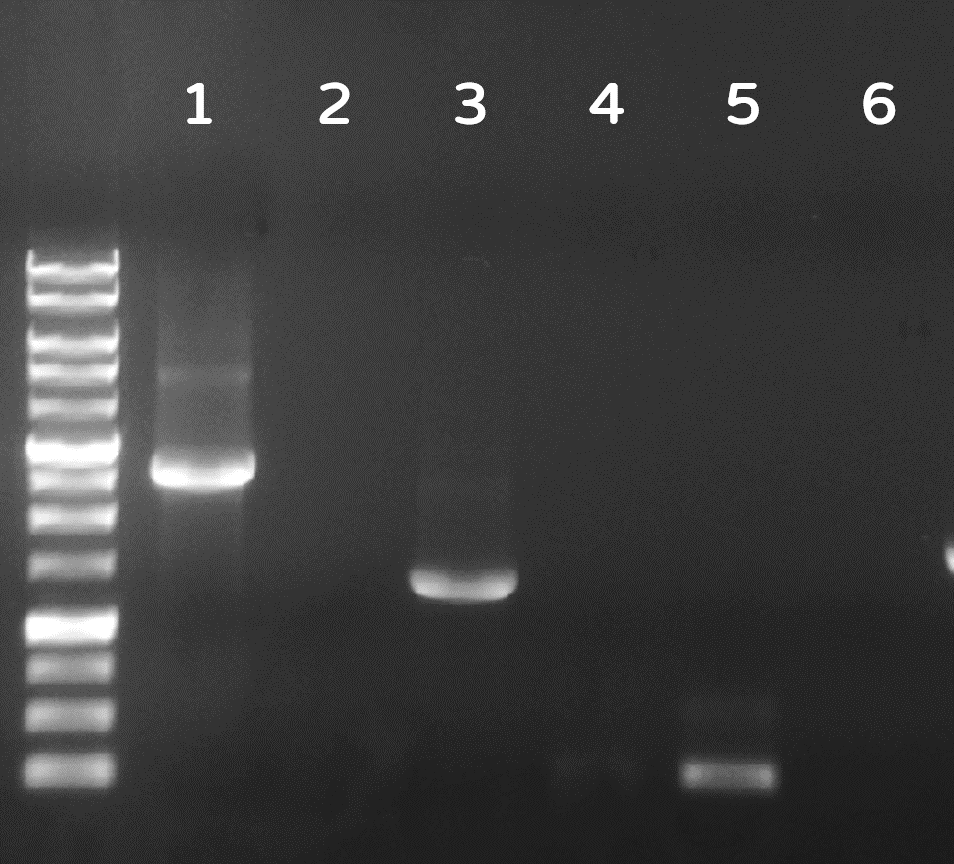
ThisDozens of plaques were screened by PCR with phi29 DNA polymerase gene-specific primers. A representative result on DNA gel electrophoresis was shown in Fig. 6, in which the successful Ф29/Tol2 insertion (Salmonella phage::Ф29 DNA polymerase, or #ST1::Ф29 for short) can be amplified by PCR with either Tol2 transposable element-specific primers or phi29 DNA polymerase-specific primers, compared to no PCR products from wild-type Salmonella phage #ST1, showing the success of our Salmonella phage engineering with phi29 DNA polymerase gene.
Figure 6 | Salmonella genome were checked by PCR with Tol2 transposable element-specific primers (lanes 1, 2) or with phi29 DNA polymerase gene-specific primer set 1 (lanes 3, 4) or set 2 (lane 5, 6). The odd numbers refer to Salmonella phage::Ф29 DNA polymerase (#ST1::Ф29), and the even numbers refer to wild-type Salmonella phage #ST1. The gel electrophoresis was performed on a 1% agarose gel with a 1kb DNA ladder.
Salmonella Detection
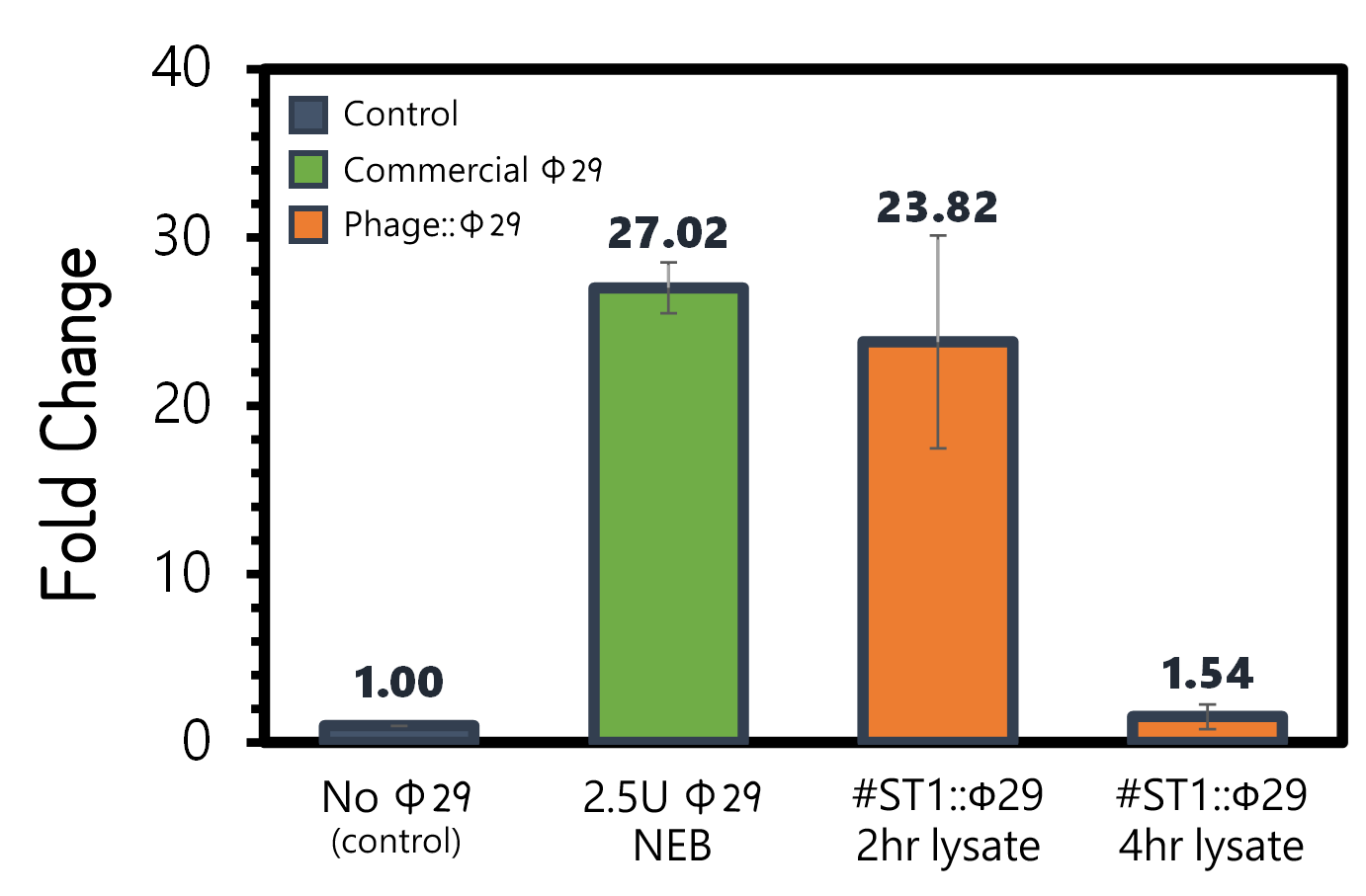
ThisAn overnight culture of Salmonella Typhimurium LT2 (~109 cells/ml) were infected by the engineered reporter phage #ST1::Ф29 at MOI=0.1 to produce phi29 DNA polymerase. The lysates were collected after 2 hr or 4 hr of treatment, and then subjected to RCA test. The lysate of phage-infected Salmonella at 2 hr can induce strong RCA reaction (24-fold change) comparable to 2.5U of a commercial phi29 DNA polymerase (NEB) in Fig. 7. Surprisingly, the lysate at 4 hr can not trigger any signal in RCA, suggesting a quick decay of phi29 DNA polymerase in the phage-infected bacterial lysates.
Figure 7 | RCA assay with NEB phi29 DNA polymerase or the Salmonella (109 cells/ml) lysates infected by #ST1::Ф29 at MOI=1 which were collected at 2 hr or 4 hr post infection. The fold changes were calculated by the fluorescence intensity of EvaGreen DNA binding of RCA materials without phi29 DNA polymerase as a background control. RCA was performed at 30°C for 1 hr.
Detection Time
ThisWe are wondering whether the time of bacterial lysis by phage infection affects the stability of phi29 DNA polymerase protein. An isolated phage may be featured by a latent time (phage generation time in a bacterial cell) and a burst size (numbers of phage produced per bacterial cell). And the latent time and burst size are in a relationship in terms of bacterial density[9] and MOI of phage infection[10].
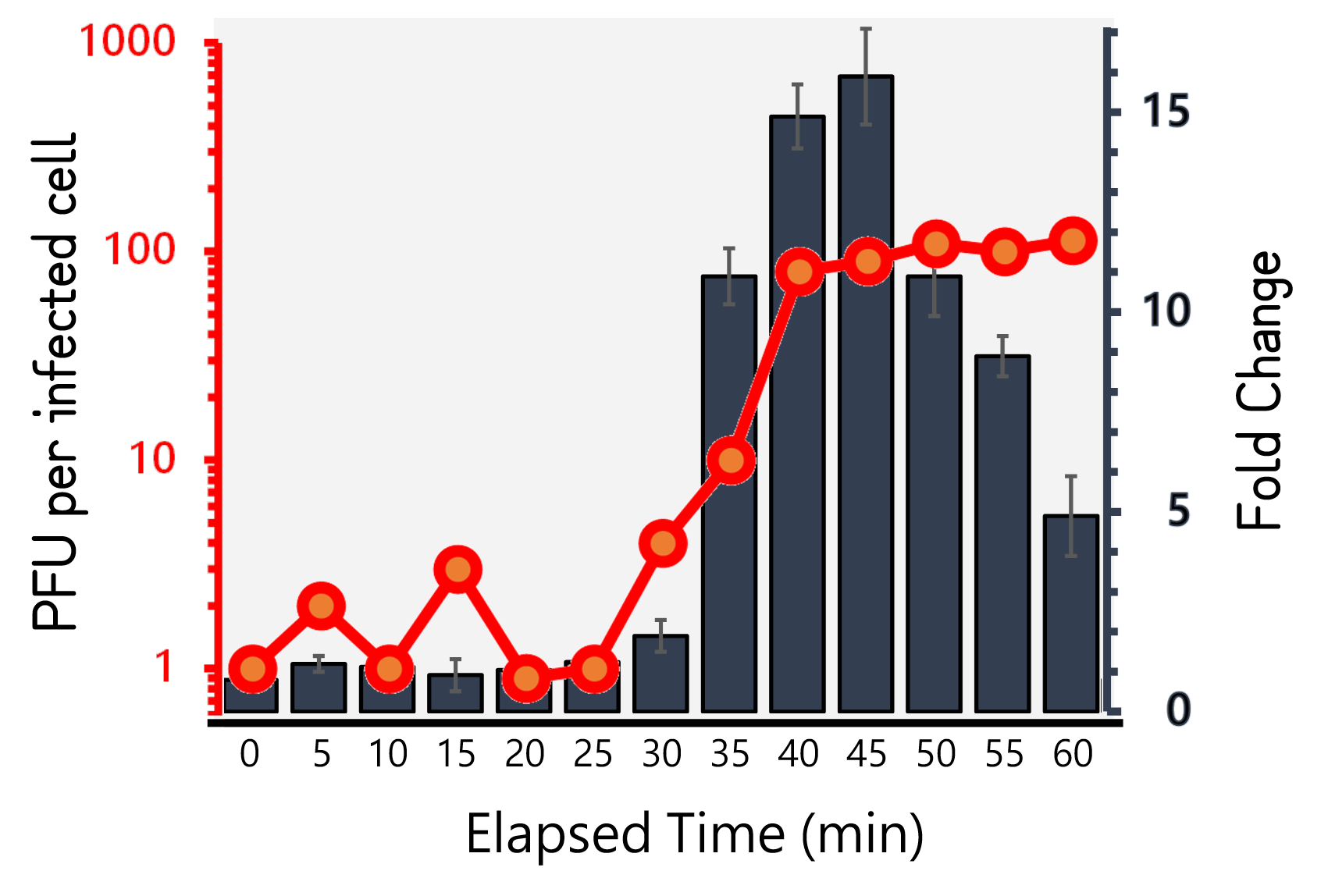
ThisTherefore, we performed the experiment to figure out the latent time and burst size of our Salmonella phage #ST1::Ф29 and the time course of RCA signals during phage infection in Salmonella. 105 cells/ml of Salmonella were infected by phage #ST1::Ф29 at MOI=1. The lysates collected by an interval of 5 min until 60 min and subjected to plaque assays and RCA test. As Fig. 8 demonstrated, the phages were released at around 40 min (latent time) to a plateau level with a burst size of average 98.4±14. Interestingly, the RCA signal increased dramatically at 35 min, achieved a high level around 40-45 min, and dropped significantly thereafter, that are consistent with our speculation of the correlation between phage lysis time (latent time) and phi29 DNA polymerase protein functionality.
Figure 8 | Salmonella phage #ST1::Ф29 latent time (min) and burst size (PFU per infected cell, the left Y axis) at MOI=1, and the relationship to RCA assay (fold change, the right Y axis). Phage-infected Salmonella lysates were harvested for 1 hour at an interval of 5 min. The lysates were subjected to plaque assays and RCA reaction followed by stained with EvaGreen dye. The burst sizes were counted by numbers of plaques. The RCA signal were read at Ex/Em=500/530 nm and divided by the background level without phi29 DNA polymerase.
Detection Limit with Our Hardware
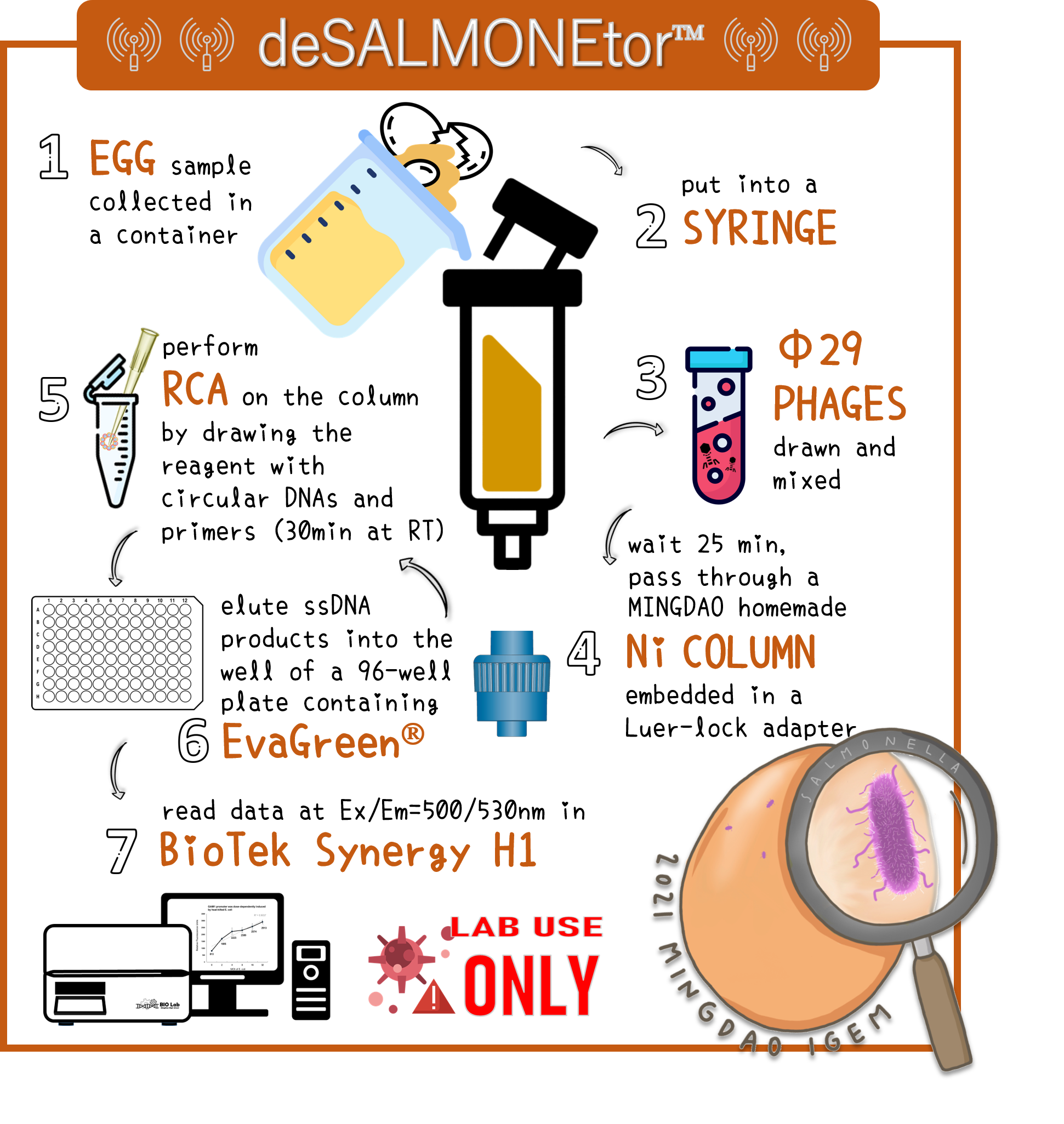
ThisImagine an application of real Salmonella diagnosis in a food or drink. A contaminated sample collected in a large volume may find 1-100 CFU/ml of bacteria[11]. Large volume and bacterial density are key parameters to affect the result of Salmonella detection with our product.
ThisTo overcome the large volume of a sample, we were inspired by Nickel column purification, in which His-tagged phi29 DNA polymerase were bound. Therefore, we think this method may enrich the His-phi29 DNA polymerase from the sample of large volume. Go to our page of (HARDWARE) for such a design. And the schematic diagram was shown here.
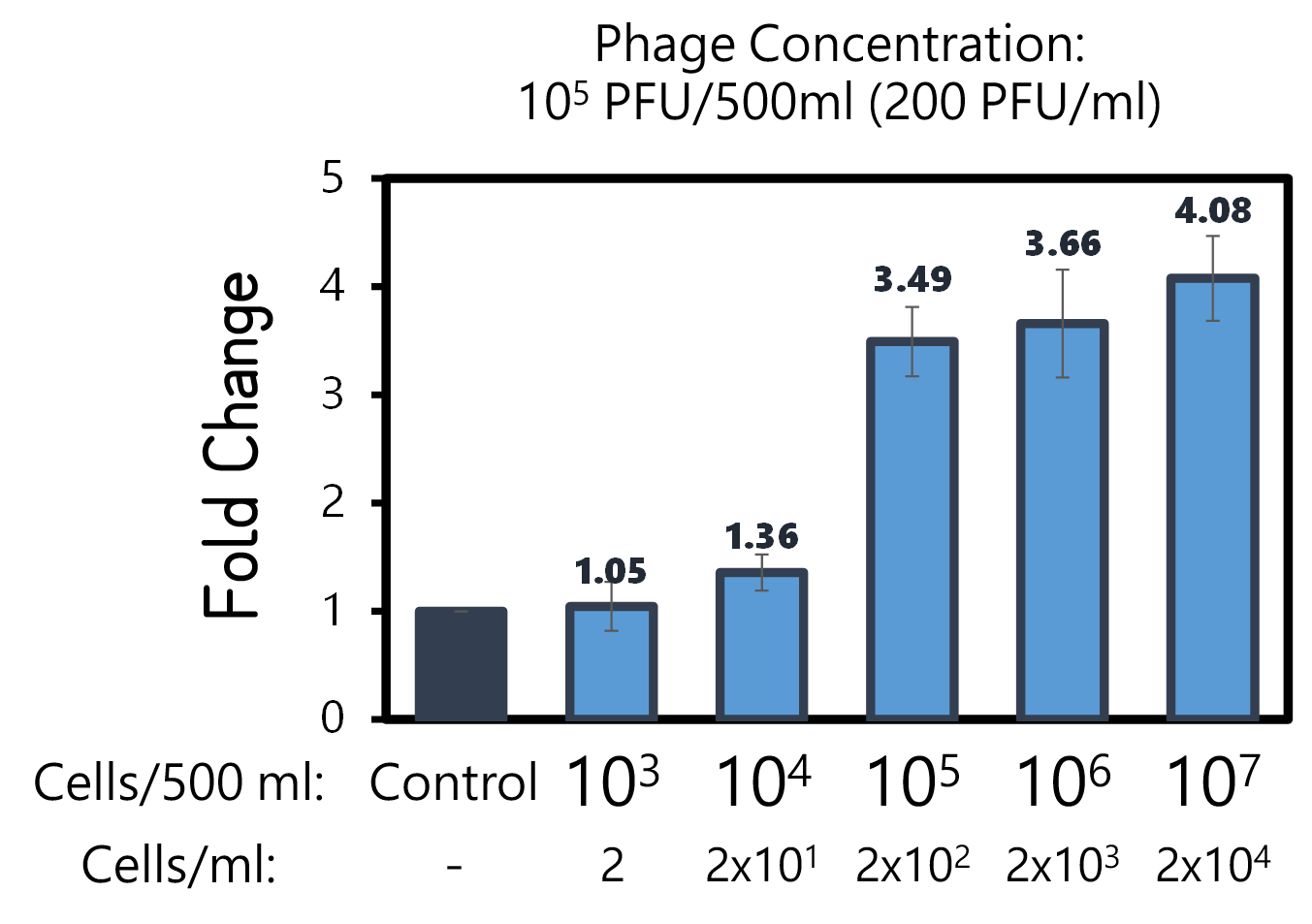
ThisTo examine the feasibility, we made a serial dilution of Salmonella from 107 to 103 cells in a beaker of 500ml water and prepared the water without bacteria as a control. The water were mixed with Salmonella phage #ST1::Ф29 at the concentration of 105 PFU/500ml at room temperature for 25 min. The 3D-printed Luer locker embedded a mini Ni-column was assembled onto a syringe, followed by repeatedly drawing up and pushing back the water in order to pass through the Ni-column. Then, the RCA materials were drawn onto the Ni-column. If His-phi29 DNA polymerase is present, the RCA reaction may be turned on. After 30 min incubation for RCA reaction, the mixtures were push back into a well of a 96-well black plate containing EvaGreen Dye in a total volume of 50 μl. The fluorescence signals were measured at Ex/Em=500/530 nm. Significant RCA signals began to appear in 2x102 bacterial cells/ml (Fig. 9). 20 cells/ml can be detected with a slight enhanced signal that is able to be distinguished from the background. However, we can’t measure the cell density under 10 cells/ml of a liquid to be examined.
Figure 9 | Salmonella test at various concentrations between 2-2x104 cells/ml in 500ml water with engineered Salmonella phage carrying His-phi29 DNA polymerase gene at the concentration of 200 PFU/ml. RCA was performed on the embedded Ni-column in a 3D-printerd Luer lock adapter. The amplified DNAs were stained with EvaGreen Dye and measured at Ex/Em=500/530 nm in a microplate reader (BioTek Synergy H1).
COMPARISON WITH CURRENT METHODS
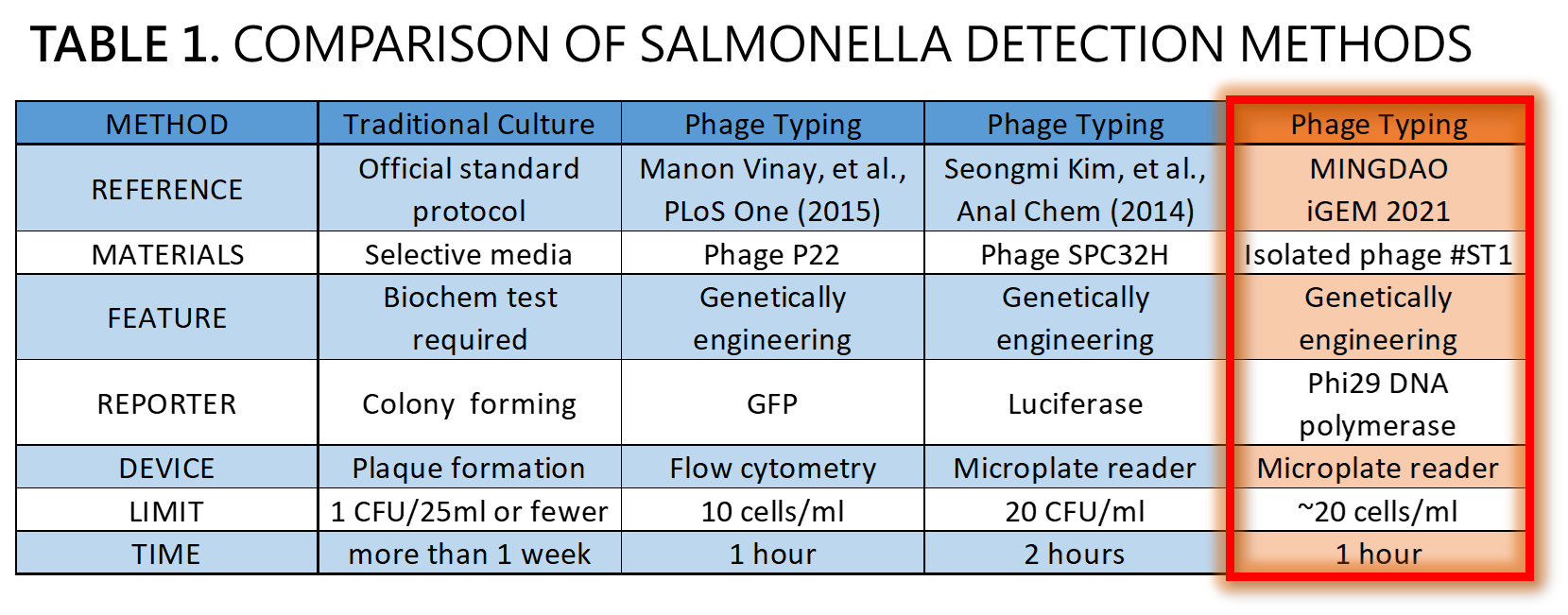
ThisWe demonstrated a proof of concept that it is possible to detect Salmonella by an isolated genetically engineered Salmonella-specific phage carrying phi29 DNA polymerase gene. The His-tagged phi29 DNA polymerase can be enriched from a large volume of a sample onto the homemade mini Ni-column in a Luer-lock adaptor format, where isothermal RCA may be triggered in the presence of the extraordinarily processive phi29 DNA polymerase at room temperature in a time as short as within 30 min. This</span>Bacteriophages possess features that can produce large amounts of phage progeny (the burst size, usually dozens to hundreds PFU per infected bacterium) to release by killing bacterial host in a short time (the latent time, usually 20 min to 1 hr). We mathematically modeled the latent time (min) as a function of cell density (cells/ml) and applied it to simulate a possible real condition using reporter phage to detect Salmonella in a poisoning case. Go to our MODEL page for a detail. We predicted the optimal bacterial lysis time (latent time) for RCA is between 16.545 and 24.427 min for 1 to 100 cells/ml of bacteria. Finally, we developed a hardware of 3D-printed Luer-lock adapter containing mini Ni-column. We used it to measure the Salmonella in 500 ml of water. We can detect 200 bacterial cells/ml within 1 hour. About 20 cells/ml of bacteria may be the most probable limit of detection in our device that generated a slight but distinguishable signal compared to the background level of no bacteria control. Compared to traditional Salmonella tests and published engineered reporter phage, we think our product is better than traditional method in term of test time, and our product is comparable and competitive in terms of test limit and time to the current designer phages carrying reporter genes[12][13][14] (Table 1).
Reference
- ↑ Johne R, Müller H, Rector A, van Ranst M, Stevens H. Rolling-circle amplification of viral DNA genomes using phi29 polymerase. Trends Microbiol. 2009 May;17(5):205-11. doi: 10.1016/j.tim.2009.02.004.
- ↑ Yue S, Li Y, Qiao Z, Song W, Bi S. Rolling Circle Replication for Biosensing, Bioimaging, and Biomedicine. Trends Biotechnol. 2021 Mar 11:S0167-7799(21)00039-1. doi: 10.1016/j.tibtech.2021.02.007.
- ↑ Urasaki A, Morvan G, Kawakami K. Functional dissection of the Tol2 transposable element identified the minimal cis-sequence and a highly repetitive sequence in the subterminal region essential for transposition. Genetics. 2006 Oct;174(2):639-49. doi: 10.1534/genetics.106.060244.
- ↑ Khattak S, Murawala P, Andreas H, Kappert V, Schuez M, Sandoval-Guzmán T, Crawford K, Tanaka EM. Optimized axolotl (Ambystoma mexicanum) husbandry, breeding, metamorphosis, transgenesis and tamoxifen-mediated recombination. Nat Protoc. 2014 Mar;9(3):529-40. doi: 0.1038/nprot.2014.040.
- ↑ Marshall R, Noireaux V. Synthetic Biology with an All E. coli TXTL System: Quantitative Characterization of Regulatory Elements and Gene Circuits. Methods Mol Biol. 2018;1772:61-93. doi: 10.1007/978-1-4939-7795-6_4.
- ↑ Tinafar A, Jaenes K, Pardee K. Synthetic Biology Goes Cell-Free. BMC Biol. 2019 Aug 8;17(1):64. doi: 10.1186/s12915-019-0685-x.
- ↑ 7.0 7.1 Shin J, Jardine P, Noireaux V. Genome replication, synthesis, and assembly of the bacteriophage T7 in a single cell-free reaction. ACS Synth Biol. 2012 Sep 21;1(9):408-13. doi: 10.1021/sb300049p.
- ↑ Rustad M, Eastlund A, Jardine P, Noireaux V. Cell-free TXTL synthesis of infectious bacteriophage T4 in a single test tube reaction. Synth Biol (Oxf). 2018 Jan 22;3(1):ysy002. doi: 10.1093/synbio/ysy002.
- ↑ Abedon ST. Selection for bacteriophage latent period length by bacterial density: A theoretical examination. Microb Ecol. 1989 Sep;18(2):79-88. doi: 10.1007/BF02030117.
- ↑ Atel, IR, and K. K. Rao. Bacteriophage burst size as a function of multiplicity of infection. Current Science 1984 53(4): 198–200.
- ↑ Gwimbi P, George M, Ramphalile M. Bacterial contamination of drinking water sources in rural villages of Mohale Basin, Lesotho: exposures through neighbourhood sanitation and hygiene practices. Environ Health Prev Med. 2019 May 15;24(1):33. doi: 10.1186/s12199-019-0790-z.
- ↑ Smartt AE, Ripp S. Bacteriophage reporter technology for sensing and detecting microbial targets. Anal Bioanal Chem. 2011 May;400(4):991-1007. doi: 10.1007/s00216-010-4561-3
- ↑ Vinay M, Franche N, Grégori G, Fantino JR, Pouillot F, Ansaldi M. Phage-Based Fluorescent Biosensor Prototypes to Specifically Detect Enteric Bacteria Such as E. coli and Salmonella enterica Typhimurium. PLoS One. 2015 Jul 17;10(7):e0131466. doi: 10.1371/journal.pone.0131466.
- ↑ Kim S, Kim M, Ryu S. Development of an engineered bioluminescent reporter phage for the sensitive detection of viable Salmonella typhimurium. Anal Chem. 2014 Jun 17;86(12):5858-64. doi: 10.1021/ac500645c.
Note: The map was generated and sponsored by SnapGene.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI site found at 765
Illegal SapI.rc site found at 1163