Difference between revisions of "Part:BBa K2323004"
(→Contribution by Team IISER_Kolkata 2021) |
|||
| Line 108: | Line 108: | ||
]] | ]] | ||
| − | '''References''' | + | '''References'''<br> |
| − | 1) Niewoehner, O., Garcia-Doval, C., Rostøl, J. et al. Type III CRISPR–Cas systems produce cyclic oligoadenylate second messengers. Nature 548, 543–548 (2017). https://doi.org/10.1038/nature23467 | + | 1) Niewoehner, O., Garcia-Doval, C., Rostøl, J. et al. Type III CRISPR–Cas systems produce cyclic oligoadenylate second messengers. Nature 548, 543–548 (2017). https://doi.org/10.1038/nature23467<br> |
| − | 2) Jonathan S. Gootenberg, Omar O. Abudayyeh, A Max J. Kellner, A Julia Joung, A James J. Collins, A Feng Zhang et al. T Multiplexed and portable nucleic acid detection platform with Cas13, Cas12a, and Csm6. 2018 Science P439-444 V 360 N 6387 doi:10.1126/science.aaq0179 | + | 2) Jonathan S. Gootenberg, Omar O. Abudayyeh, A Max J. Kellner, A Julia Joung, A James J. Collins, A Feng Zhang et al. T Multiplexed and portable nucleic acid detection platform with Cas13, Cas12a, and Csm6. 2018 Science P439-444 V 360 N 6387 doi:10.1126/science.aaq0179<br> |
| − | 3) Kellner, M.J., Koob, J.G., Gootenberg, J.S. et al. SHERLOCK: nucleic acid detection with CRISPR nucleases. Nat Protoc 14, 2986–3012 (2019). https://doi.org/10.1038/s41596-019-0210-2 | + | 3) Kellner, M.J., Koob, J.G., Gootenberg, J.S. et al. SHERLOCK: nucleic acid detection with CRISPR nucleases. Nat Protoc 14, 2986–3012 (2019). https://doi.org/10.1038/s41596-019-0210-2 <br> |
| − | 4) http://2017.igem.org/Team:Munich | + | 4) http://2017.igem.org/Team:Munich <br> |
| − | 5) https://www.uniprot.org/uniprot/U2PSH1 | + | 5) https://www.uniprot.org/uniprot/U2PSH1 <br> |
Revision as of 22:09, 18 October 2021
Lwa Cas13a under T7 promoter with solubility tag
Uses a T7 promoter and RBS B0034 (BBa_B0034) to express Lwa Cas13a (BBa_K2323000) flanked by a 6x His-Tag to allow purification and a SUMO-tag to improve solubility. This is terminated by a Tphi terminator. The whole coding sequence is also available as BBa_K2323001.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 1768
Illegal BglII site found at 3448
Illegal BamHI site found at 144 - 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI.rc site found at 2847
Usage and Biology
Cas13a from Leptotrichia wadei (Lwa) is class 2 type VI CRISPR-Cas effector. It is capable of targeting specific ssRNA and cleaving them using its bounded crRNA as guide strand. Unlike other ssRNA CRISPR-Cas effectors, Cas13a is capable of processing the precursor crRNA (pre-crRNA) by itself, cleaving, binding the RNA sequence and then building the Cas13a:crRNA surveillance complex. One characteristic of Cas13a along with other type VI members is its ability to degrade other RNA strands regardless of their sequences, provided the protein has been activated by binding and digesting the target sequence complementary to the crRNA. This "collateral activity" makes Cas13a a powerful biological tool, as unspecific RNA degradation can be triggered on command by providing the target RNA.
Plasmid composition
The Biobrick contains a promoter, RBS B0034 and Lwa-Cas13a with a His-SUMO-Tag, that allows for easy expression and purification. The SUMO-tag increases solubility and can be cleaved off with a SUMO-protease after the affinity purification.
Cloning, Expression and Purification
Cloning
We ordered Lwa in 2 gblocks from IDT, ready for Golden-Gate-Assembly. We ligated it into the pSB1C3-ccdB by restriction digestion of the backbone and having adequate BsaI sites within the gblocks. We confirmed the cloning by colony-PCR, analytical restriction digest and sequencing at GATC. The colony was stored as a Cryo-stock at -80 °C.

| Name | 5'-3' primers sequences |
|---|---|
| pseq-Lwa-01 | CGTTTTCTGTATGACGGCATCC |
| pseq-Lwa-02 | TCCGGATATGAGCGAACTTAAGA |
| pseq-Lwa-03 | GAGAACCTGAAGATGTTTTA |
| pseq-Lwa-04 | ATCATAGGAAAGCAGTTTG |
Expression
As the sequence was ordered codon optimized, we could express it in BL21 star. Expression was done overnight at 16 °C in 2xYT media in the pressence of 1 mM IPTG.
Purification
Characterisation
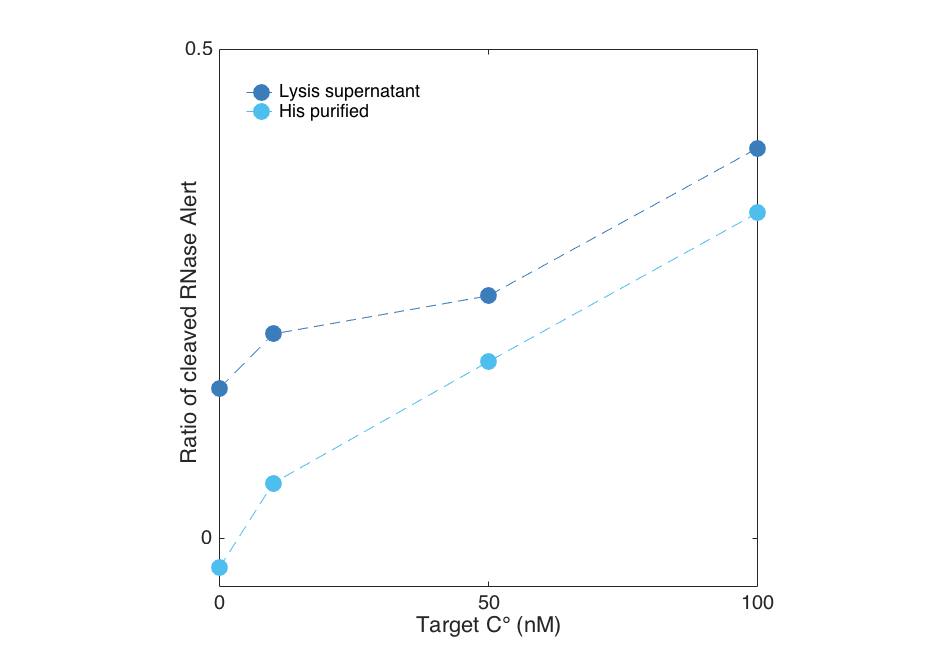
Target dependent activity of Cas13a Lwa
We show below, that Cas13a gets activated upon target binding in a concentration dependent manner. In this case, we detected a fragment of the 16S rRNA of E. coli in a plate reader experiment by using the RNaseAlert system readout. After less than 30 minutes a difference between a target containing sample and the negative control (sample without Cas13a Lwa) is visible for both the purified and the unpurified Cas13a. However, the unpurified Cas13a has a visibly larger activity at low target concentrations, and even shows cleavage at 0 nm target. This is probably attributed to residual RNases that are within the cellular lysis mix. The positive control contained RNAse A, which cleaved all RNAseAlert before the measurment could be started in the plate reader.
- Target dependent activity screen of the purified Cas13a
When plotted against each other, the previously mentioned residual activity becomes even clearer. For this reason we suggest a proper purification of the protein to get reproducible results.
Contribution by Team ZJUT_China_B 2020
Group: Team ZJUT_China_B 2020
Author: Xiaojie Zhou
Summary: We learned from literature that Cas13 protein homologues not only have target dependent activity(characterized by iGEM 2017_Munich) but also have different motif preference upon specific nucleotides and LwaCas13a protein's base preference is poly U/AU.This year,on the basis work of iGEM 2017_Munich, ZJUT_China_B further characterized the base preference of LwaCas13a protein's collateral cleavage,which may be a potential feature to achieving multivirus detecting in one pot.
Methodology
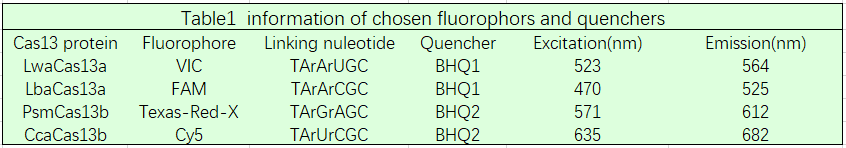
Based on our multivirus detecting goal, we designed four kind fluorescent reporters (Table 1). The linking nucleotide are AC, AU, GA, and UC respectively. The fluorophore are FAM, Cy5, Texas-Red-X and VIC respectively. This enables us detecting the corresponding extention of the cleavage. We added those 4 fluorescent reporters in one detecting pot, and got the following data.
Results
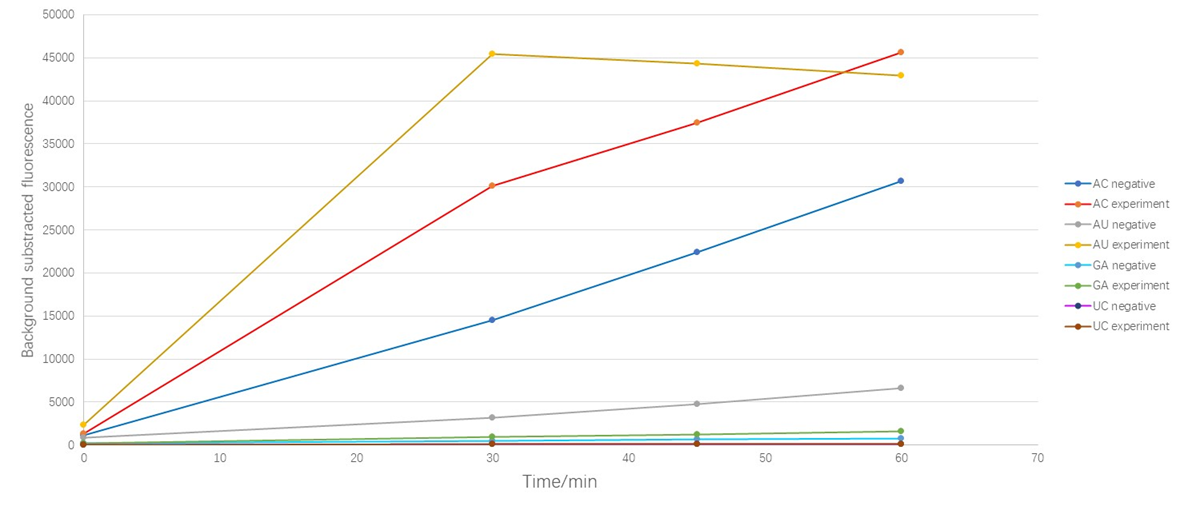
1. Because our former experiment shows that as the excitation goes on, our fluorescent dyes show a certain degree of photobleaching phenomenon, so we decrease the time of excitation. For details of photobleaching phenomenon,click ZJUT_China_B Model
2. The "negative" is the group that we didn't add target while the "experiment" is the group where we add our target.
Analysis
As shown in Figure 1, the results suggest that LwaCas13a protein could cleave AU and AC oligonucleotide even though there is no target in the detecting system which we called "the leakage acticity of Cas13 protein ". Obviously, the LwaCas13a protein shows more "leakage" upon AC while the leakage upon GA and UC are quite slight. When we added target sequence in the detecting system, the cleavage of AC obviously increased (the RFU increased 2.04 times at 30min) but the background subtracted fluorescence of AU reporter increased in a cliff like manner (the RFU increased 13. 89 times at 30min), which means that when the target was added, the protein's activity to cleave AU was activated significantly. Due to the preference appears only when the target was added, we defined this as the cleavage base preference of LwaCas13a protein, or more specifically, "the activating associated cleavage base preference " of the protein.
Contribution by Team IISER_Kolkata 2021
Group: iGEM21_IISER_Kolkata
Author: Soumi Bhattacharyya, Shubhamay Das
Summary: We learnt that Team iGEM17_Munich has performed the assay for target-dependent activity of purified Cas13a protein under different concentrations of target RNA molecules. Team ZJUT_China_B 2020 has characterized the base preference of collateral cleavage activity of LwaCas13a protein. Due to limitations in laboratory access, we have contributed more information about this part through literature survey.
New information: Zhang Lab devised the SHERLOCK technique which uses isothermal preamplification with specific activation and non-specific cleavage activity of Cas13a to detect small RNA or DNA molecules. This is a significant scientific advancement in the field of rapid nucleic acid detection used in diagnostics and biotechnological applications. LwaCas13a collateral cleavage activity results in the production of cleaved RNA products with hydroxylated 5’ ends and 2’-3’ cyclic phosphate ends. A Type III CRISPR Cas system protein called Csm6 gets efficiently activated by 2’-3’ cyclic phosphate terminated hexadenylated ends [1].
Zhang et al. have further devised SHERLOCKv2 [2] which is an advancement of the SHERLOCK technique [3]. In this method, orthogonal CRISPR enzymes Cas13a and Csm6 are multiplexed together to detect RNA molecules. SHERLOCKv2 acts by the method of initial specific activation of cas13a by target RNA molecules which then goes on to generate cleavage residues/overhangs of 2’-3’ cyclic phosphate by collateral cleavage of RNA in the sample. These overhangs further activate Csm6 which also carries out collateral cleavage. According to literature, it was found that this can be used to detect as low as 2 attomolar input RNA with a preamplification step (Eg: Recombinase Polymerase Amplification). They also devised a lateral flow detection technique where the signal of LwaCas13a is enhanced by EiCsm6(Csm6 from Enterococcus italicus) solely, without the preamplification step by RPA.


References
1) Niewoehner, O., Garcia-Doval, C., Rostøl, J. et al. Type III CRISPR–Cas systems produce cyclic oligoadenylate second messengers. Nature 548, 543–548 (2017). https://doi.org/10.1038/nature23467
2) Jonathan S. Gootenberg, Omar O. Abudayyeh, A Max J. Kellner, A Julia Joung, A James J. Collins, A Feng Zhang et al. T Multiplexed and portable nucleic acid detection platform with Cas13, Cas12a, and Csm6. 2018 Science P439-444 V 360 N 6387 doi:10.1126/science.aaq0179
3) Kellner, M.J., Koob, J.G., Gootenberg, J.S. et al. SHERLOCK: nucleic acid detection with CRISPR nucleases. Nat Protoc 14, 2986–3012 (2019). https://doi.org/10.1038/s41596-019-0210-2
4) http://2017.igem.org/Team:Munich
5) https://www.uniprot.org/uniprot/U2PSH1