Difference between revisions of "Part:BBa K3716012"
(→Results) |
(→Results) |
||
| Line 39: | Line 39: | ||
[[File:File:T--iBowu-China--2021lacI-5|thumb|center|600px|'''Figure.4. Measurement of enzyme activities and comparisons between the β-glucuronidase sequence from E. coli source (T7-bG-pet), Aspergillus oryzae source (bG14-pet), the Thermotoga maritima source (bG15-pet) and also a control group (vacant-pet). ''' ]] | [[File:File:T--iBowu-China--2021lacI-5|thumb|center|600px|'''Figure.4. Measurement of enzyme activities and comparisons between the β-glucuronidase sequence from E. coli source (T7-bG-pet), Aspergillus oryzae source (bG14-pet), the Thermotoga maritima source (bG15-pet) and also a control group (vacant-pet). ''' ]] | ||
| − | Measurement of enzyme activities can be easily achieved by using β-glucuronidase | + | Measurement of enzyme activities can be easily achieved by using β-glucuronidase to hydrolyze specific substance in a buffer and measure the hydrolysis product. Here we used Phenolphthalein-β-D-glucuronide as the substrate and after hydrolysis reactions with β-glucuronidase, free phenolphthalein will be released into the buffer. The system would show a pink to purple color after adding NaOH solution at a prepared standard concentration. The activity can also be quantitatively measured by measuring the light emission at 540 nm, which is the most intensive emission of colorant. |
| − | and | + | |
| − | + | ||
| − | + | ||
| + | In the measurement, we added a control group labeled vacant-pet, where the plasmid and the gene circuit is the same, but the β-glucuronidase is removed. The control group went through exactly the same process of incubation and induction with Iptg to provide a background reading. | ||
| + | We also compare between the β-glucuronidase sequence from E. coli source (T7-bG-pet), Aspergillus oryzae source (bG14-pet), the Thermotoga maritima source (bG15-pet) and also a control group (vacant-pet). We found the enzyme encoded by the E. coli source (T7-bG-pet) has a significantly higher enzyme activity compared to others; and the enzyme activity from Aspergillus oryzae source and Thermotoga maritima source is not stronger than the background. Previous research works reported positive enzyme activities of the β-glucuronidase enzyme from these two sources, but here in our experiment conditions these two enzymes do not display discernible performance. | ||
<b>Summary</b> | <b>Summary</b> | ||
| − | + | The gene circuit is proper for the expression of β-glucuronidase enzyme. The enzyme from E. coli source has shown relatively high activity while the expression conditions for these two enzymes would have to be further explored to achieve good catalytic hydrolysis performance. | |
| − | + | ||
| − | + | ||
Revision as of 05:46, 18 October 2021
6xHis-Beta-glucuronidase (E.coli, gusA)
6xHis-Beta-glucuronidase (E.coli, gusA)
Design
Introduction
This year team iBowu-China used this part to express the enzyme called β-glucuronidase in E. coli strain BL21(DE3) and similar variants of the strain. The designed purpose of this enzyme is to convert glycyrrhizic acid into glycyrrhetinic acid, which is the effective active ingredient of licorice.
Protocol
- Transform the plasmids into E. coli BL21(DE3)
- Pick a single colony by a sterile tip from each of the LB plates for all the experimental and control groups. Add the colony into 4ml LB medium with kanamycin. Add 1mM iPTG to all experimental groups. Incubate at 37℃ in a shaker overnight.
- Add 100 µl bacteria culture medium into a sterile 96-well plate. Measure fluorescence at 488nm excitation and 502nm absorption, and then also OD600 for normalization, with a microplate reader.
- SDS-PAGE. The cells are lysed by 150 μL 4% SDS for 5 min at room temperature, then 10 min at 95℃. Then 6x loading buffer is added. The SDS-PAGE was carried with a 12% precast polyacrylamide gel. Coomassie bright blue stain is used to observe the bands.
Results
1. Confirmation of the Gene Circuit with sfGFP
First we needed to experimentally confirm that our gene circuit would work as we had expected. We used sfGFP (Part:K515105) gene to substitute the β-glucuronidase gene in the above plasmid construction. The reconstructed plasmid was then cloned in DH5a and then extracted to be transformed into BL21(DE3). With the induction Iptg added, we can clearly measure strong signal of green fluorescence light. The intensity is about 100 fold stronger than the background, which is obtained by measuring the same bacteria culture but without Iptg induction. This result confirm that with Iptg induction, our gene circuit works normally and can be used for expression of bG enzyme.
2. Successful Expression of the Protein with SDS-PAGE
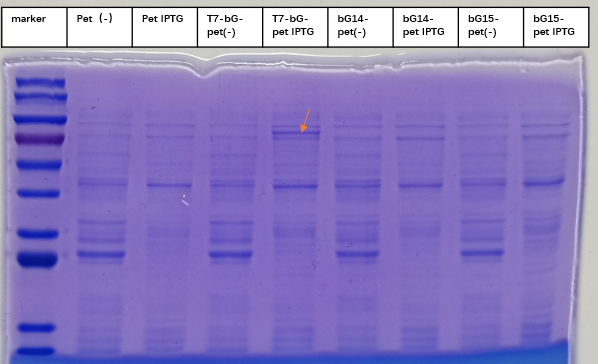
To express the enzyme β-glucuronidase, the plasmid was clone in DH5a and then extracted to transform BL21(DE3). Iptg was added at 1mM at OD600=0. The medium was incubated at 37 degree Celsius with shaking overnight, and then taken to SDS-PAGE experiment to check for the expression of the enzyme. This enzyme, which we named T7-bG-pet, was expected to have a size about 67 kDa, and the SDS-PAGE result showed the successful expression of the protein at the expected size band (marked by a red arrow on the figure). We included a control group of vacant-pet28a, of which the only difference is there the β-glucuronidase sequence is removed. Control groups where there is no Iptg induction for the same plasmid and control groups of vacant-pet28a (labeled Pet) showed no intrinsic protein exists in the expected bands.
3. Measurement of the Function of the Enzyme
Measurement of enzyme activities can be easily achieved by using β-glucuronidase to hydrolyze specific substance in a buffer and measure the hydrolysis product. Here we used Phenolphthalein-β-D-glucuronide as the substrate and after hydrolysis reactions with β-glucuronidase, free phenolphthalein will be released into the buffer. The system would show a pink to purple color after adding NaOH solution at a prepared standard concentration. The activity can also be quantitatively measured by measuring the light emission at 540 nm, which is the most intensive emission of colorant.
In the measurement, we added a control group labeled vacant-pet, where the plasmid and the gene circuit is the same, but the β-glucuronidase is removed. The control group went through exactly the same process of incubation and induction with Iptg to provide a background reading.
We also compare between the β-glucuronidase sequence from E. coli source (T7-bG-pet), Aspergillus oryzae source (bG14-pet), the Thermotoga maritima source (bG15-pet) and also a control group (vacant-pet). We found the enzyme encoded by the E. coli source (T7-bG-pet) has a significantly higher enzyme activity compared to others; and the enzyme activity from Aspergillus oryzae source and Thermotoga maritima source is not stronger than the background. Previous research works reported positive enzyme activities of the β-glucuronidase enzyme from these two sources, but here in our experiment conditions these two enzymes do not display discernible performance.
Summary The gene circuit is proper for the expression of β-glucuronidase enzyme. The enzyme from E. coli source has shown relatively high activity while the expression conditions for these two enzymes would have to be further explored to achieve good catalytic hydrolysis performance.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]


