Difference between revisions of "Part:BBa K3716012"
(→Design) |
(→Design) |
||
| Line 6: | Line 6: | ||
== Design == | == Design == | ||
| − | [[File:T--iBowu-China--2021bG-1|thumb|center|600px|'''Figure.1. The plasmid constructions used for the expression of this part. This part encodes an enzyme β-glucuronidase.''' ]] | + | [[File:T--iBowu-China--2021bG-1.png|thumb|center|600px|'''Figure.1. The plasmid constructions used for the expression of this part. This part encodes an enzyme β-glucuronidase.''' ]] |
<br/> | <br/> | ||
Revision as of 04:34, 18 October 2021
6xHis-Beta-glucuronidase (E.coli, gusA)
6xHis-Beta-glucuronidase (E.coli, gusA)
Design
Introduction
This year team iBowu-China used this part to express the enzyme called β-glucuronidase in E. coli strain BL21(DE3) and similar variants of the strain. The designed purpose of this enzyme is to convert glycyrrhizic acid into glycyrrhetinic acid, which is the effective active ingredient of licorice.
Protocol
- Transform the plasmids into E. coli BL21(DE3)
- Pick a single colony by a sterile tip from each of the LB plates for all the experimental and control groups. Add the colony into 4ml LB medium with kanamycin. Add 1mM iPTG to all experimental groups. Incubate at 37℃ in a shaker overnight.
- Add 100 µl bacteria culture medium into a sterile 96-well plate. Measure fluorescence at 488nm excitation and 502nm absorption, and then also OD600 for normalization, with a microplate reader.
IV Results
sfGFP
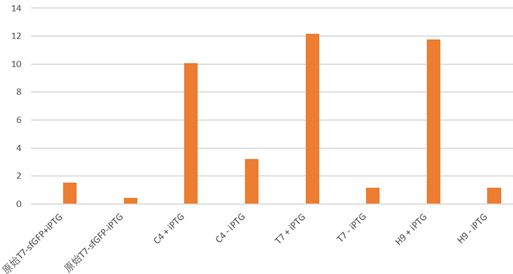
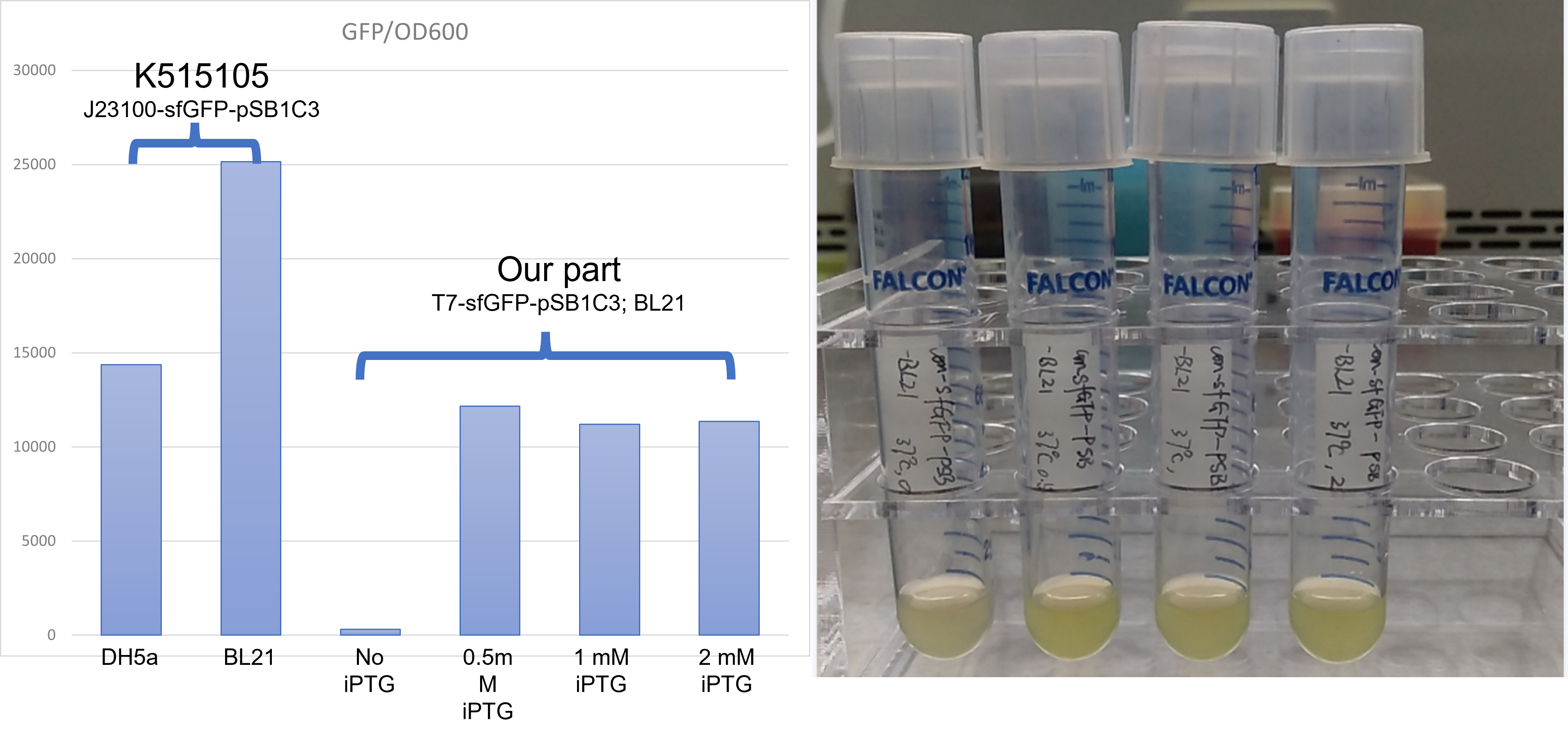
Under the condition that there exists the part LacI, and there also exists LacO in the upstream gene of sfGFP, Iptg induction can control the switch on and off of the expression of green fluorescence protein sfGFP.
The plot and photo demonstrate that 0.5nM Iptg added into the medium is sufficient to induce the expression of sfGFP with the existence of lacI part.
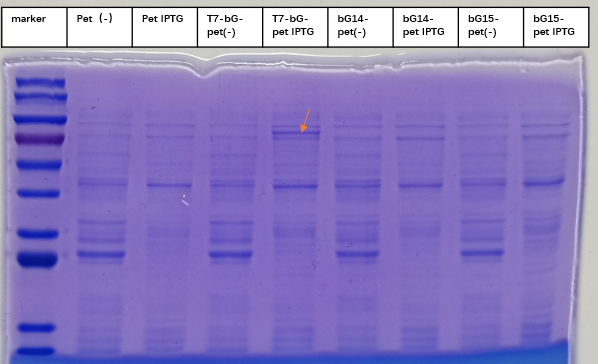
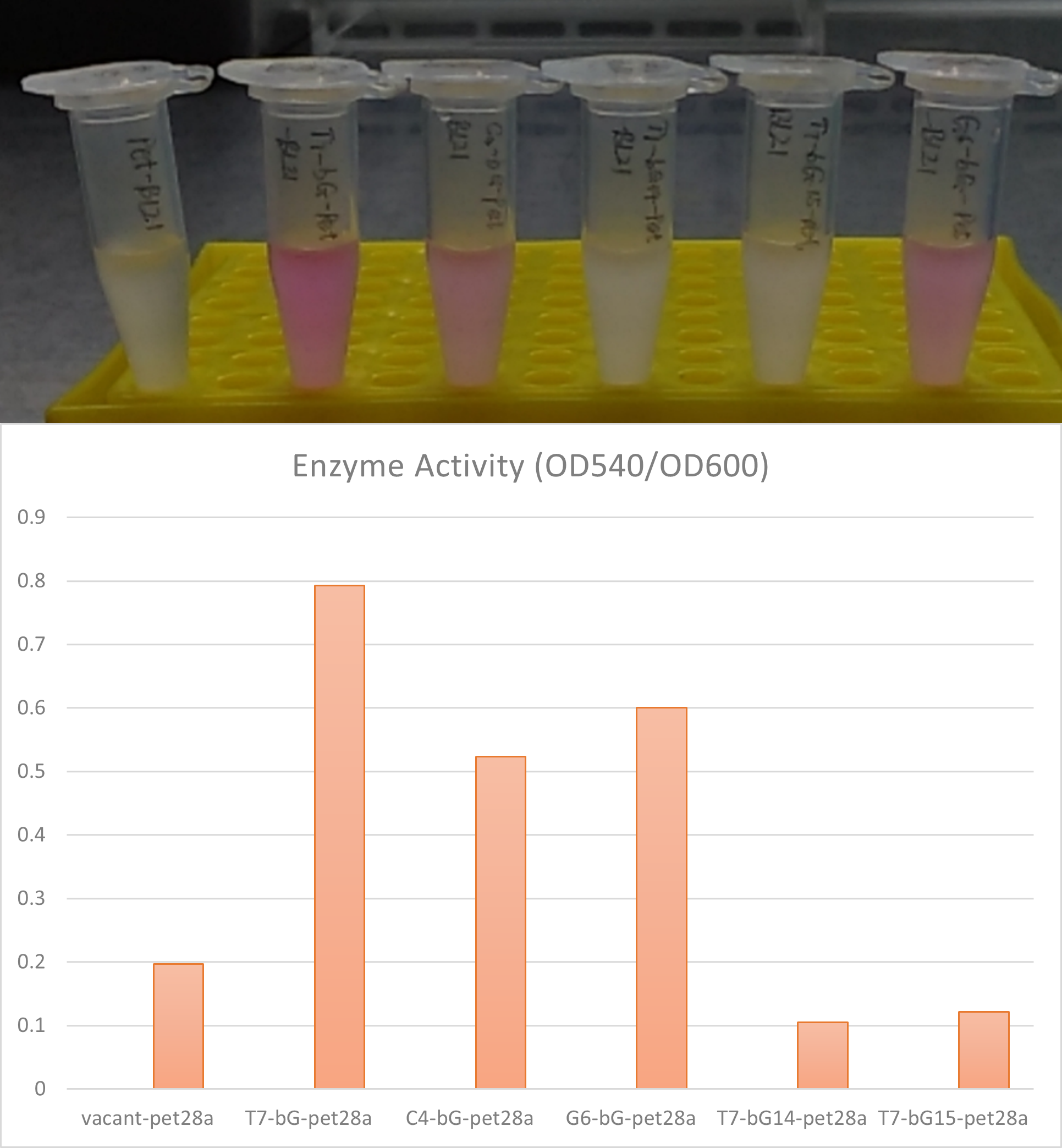
Enzyme activity of β-glucuronidase
In the scenario where there is LacI in the plasmid and also where there exists LacO in the upstream gene of β-glucuronidase, this enzyme can be expressed in E. coli BL21 with Iptg induction. The enzymic activities and therefore the catalytic capabilities of the cells and the bacteria culture can therefore be controlled with different concentrations of induction substance.
Summary LacI can effectively work together with LacO to control gene expression in E. coli BL21(DE3). Our results confirmed 0.5 to 2mM Iptg can be effective and sufficient to release the suppression of gene expression exerted by LacI protein.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]