Difference between revisions of "Part:BBa K3989010"
| Line 16: | Line 16: | ||
[[File:omp8 OMVs on col-0.jpeg|500px]] | [[File:omp8 OMVs on col-0.jpeg|500px]] | ||
| − | Figure 2. engineered < | + | Figure 2. Evaluation of engineered <I>E. coli</i> omp8 OMVs' immunogenicities by SGI assay. |
[[File:tolB OMVs on col-0.jpeg|500px]] | [[File:tolB OMVs on col-0.jpeg|500px]] | ||
| − | Figure 3. engineered < | + | Figure 3. Evaluation of engineered <I>E. coli tolB</i> OMVs' immunogenicities by SGI assay. |
| − | ROS burst assay is also used to test the immunogenicities of engineered OMVs, which shows how | + | ROS (reactive oxygen species) burst assay is also used to test the immunogenicities of engineered OMVs, which shows how plant immune system responds to OMV treatments within one to two hours. Our results show that only ClyA-elf18 ([[Part:BBa K3989010]]) OMVs, but not Cly-flg22 OMVs, can trigger significantly higher ROS production than ClyA-only OMVs. |
[[File:ROS overall.png|700px]] | [[File:ROS overall.png|700px]] | ||
| − | Figure 4. | + | Figure 4. Evaluation of engineered OMVs' immunogenicities by ROS burst assay. |
<!-- Add more about the biology of this part here | <!-- Add more about the biology of this part here | ||
Revision as of 09:45, 17 October 2021
plant immune elicitor flg22 fused with ClyA for OMV display
In this construct, outer membrane-associated protein ClyA (Part:BBa_K811000) is used to display the plant immune elicitor flg22 (Part:BBa_K3286138) to the surface of bacterial outermembrane vesicles (OMVs) to trigger plant immune responses. To make flg22 better recognized by plant immune receptor FLS2, we put mutiple linkers and a 3×FLAG tag between ClyA and flg22 to minimize the steric hindrance.
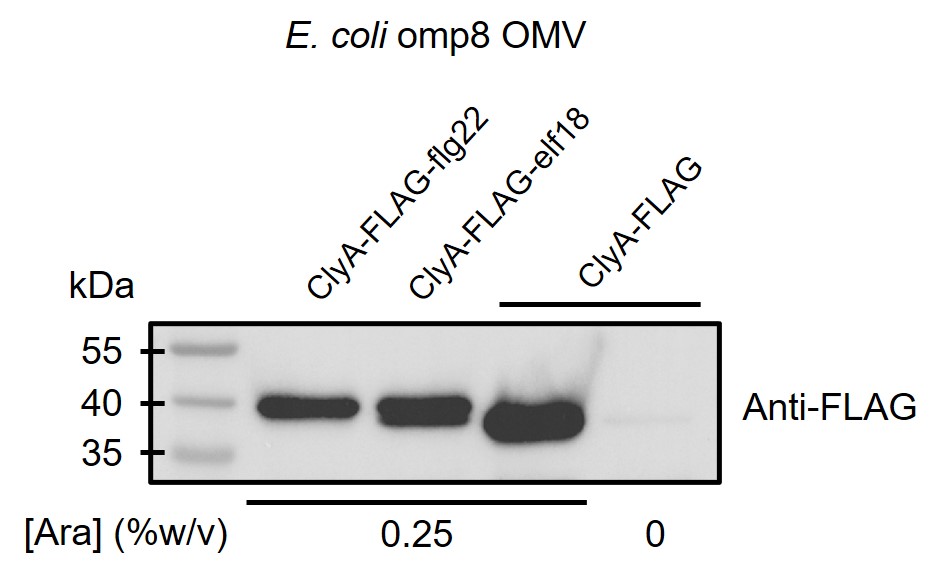
By doing western blot using anti-FLAG antibody, we proved that ClyA-elicitor fusion proteins indeed exist in OMVs (Figure 1).
Figure 1. ClyA protein with or without elicitor fused exists in outer membrane vesicles of E. coli omp8 strain.
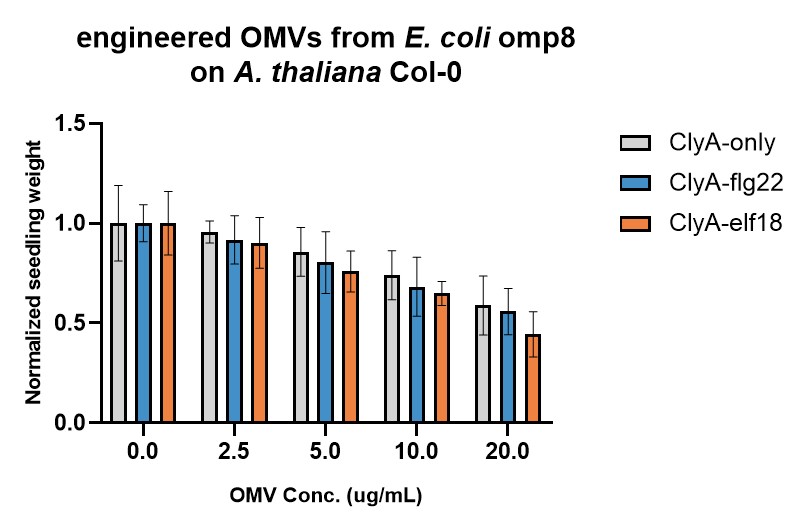
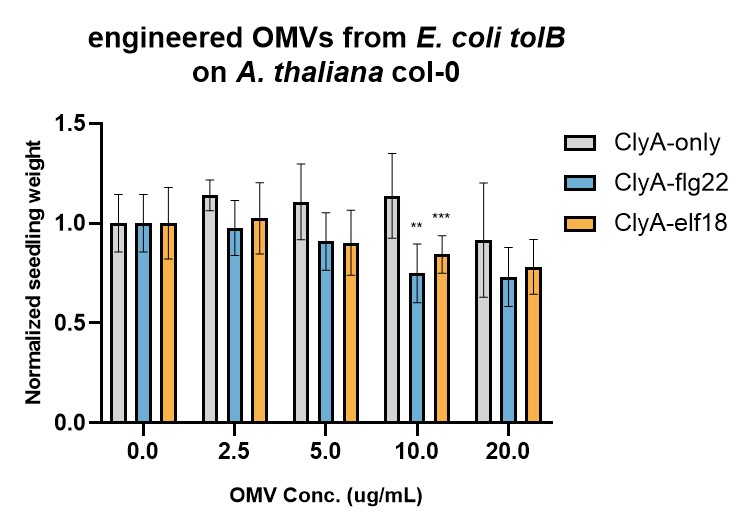
The immunogenicities of engineered OMVs were tested by Seedling Growth Inhibition (SGI) assay (Figure 2 & 3). In SGI assay, lower seedling weight indicates that higher immune response is triggered (due to the trade-off between immunity and growth). Two E. coli strains were used, omp8 (Figure 2) and tolB (Figure 3), which produce high and low immunogenic OMVs, respectively. For both strains, ClyA-flg22 and ClyA-elf18 OMVs seem to trigger stronger immune responses than OMVs containing only ClyA. However, the enhancement effect is more significant for tolB OMVs than omp8 OMVs. The reason might be that natural omp8 OMVs can already trigger strong immune responses, obscuring the additional immune responses triggered by flg22 or elf18.
Figure 2. Evaluation of engineered E. coli omp8 OMVs' immunogenicities by SGI assay.
Figure 3. Evaluation of engineered E. coli tolB OMVs' immunogenicities by SGI assay.
ROS (reactive oxygen species) burst assay is also used to test the immunogenicities of engineered OMVs, which shows how plant immune system responds to OMV treatments within one to two hours. Our results show that only ClyA-elf18 (Part:BBa K3989010) OMVs, but not Cly-flg22 OMVs, can trigger significantly higher ROS production than ClyA-only OMVs.
Figure 4. Evaluation of engineered OMVs' immunogenicities by ROS burst assay.
Sequence and Features
- 10INCOMPATIBLE WITH RFC[10]Illegal PstI site found at 1105
- 12INCOMPATIBLE WITH RFC[12]Illegal PstI site found at 1105
- 21COMPATIBLE WITH RFC[21]
- 23INCOMPATIBLE WITH RFC[23]Illegal PstI site found at 1105
- 25INCOMPATIBLE WITH RFC[25]Illegal PstI site found at 1105
Illegal NgoMIV site found at 1099
Illegal AgeI site found at 1063 - 1000COMPATIBLE WITH RFC[1000]