Difference between revisions of "Part:BBa K3886003"
| Line 8: | Line 8: | ||
===Usage and Biology/Characterisation=== | ===Usage and Biology/Characterisation=== | ||
| + | [[Image:Bielefeld2012_ECOL3LFermentation.jpg|450px|thumb|left|'''Figure 1:''' Fermentation of ''E. coli'' KRXwith <partinfo>BBa_K863005</partinfo> (ECOL) in an Infors Labfors Bioreactor, scale: 3 L, [http://2012.igem.org/Team:Bielefeld-Germany/Protocols/Materials#Autoinduction_medium autoinduction medium] + 60 µg/mL chloramphenicol, 37 °C, pH 7, agitation on cascade to hold pO<sub>2</sub> at 50 %, OD<sub>600</sub> measured every 30 minutes.]] | ||
| + | <p align="justify"> | ||
| + | After the positive measurement of activity of ECOL we made a scale-up and fermented ''E. coli'' KRX with <partinfo>BBa_K863005</partinfo> in an Infors Labfors fermenter with a total volume of 3 L. Agitation speed, pO<sub>2</sub> and OD<sub>600</sub> were determined and illustrated in Figure 1. The exponential phase started after 1.5 hours of cultivation. The cell growth caused a decrease in pO<sub>2</sub>. After 2 hours of cultivation the agitation speed increased up to 629 rmp (5.9 hours) to hold the minimal pO<sub>2</sub> level of 50 %. Then, after 4 hours there was a break in cell growth due to induction of protein expression. The maximal OD<sub>600</sub> of 2.78 was reached after 5 hours. In comparison to ''E. coli'' KRX (OD<sub>600,max</sub> =4.86 after 8.5 hours) and to ''E. coli'' KRX with <partinfo>BBa_K863000</partinfo> (OD<sub>600,max</sub> =3.53 after 10 hours, time shift due to long lag phase) the OD<sub>600 max</sub> is lower. In the following hours, the OD<sub>600</sub> and the agitation speed decreased and the pO<sub>2</sub> increased, which indicates the death phase of the cells. This is caused by the cell toxicity of ECOL (reference: [http://www.dbu.de/OPAC/ab/DBU-Abschlussbericht-AZ-13191.pdf DBU final report]). Hence, cells were harvested after 12 hours. | ||
| + | </p> | ||
<span class='h3bb'>Sequence and Features</span> | <span class='h3bb'>Sequence and Features</span> | ||
Revision as of 20:56, 14 October 2021
Caffeine Sensor
A Caffeine Sensor
Usage and Biology/Characterisation
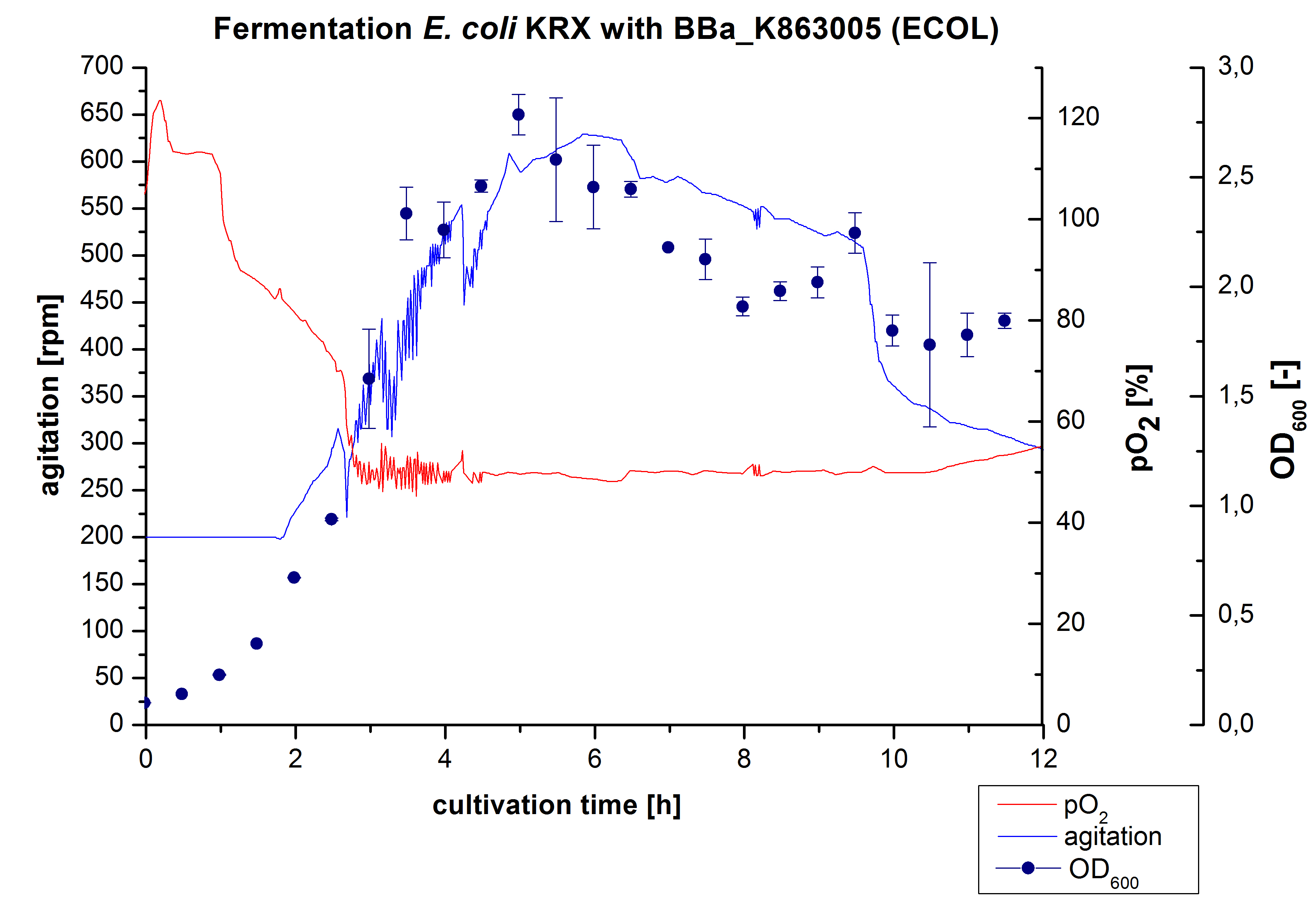
After the positive measurement of activity of ECOL we made a scale-up and fermented E. coli KRX with BBa_K863005 in an Infors Labfors fermenter with a total volume of 3 L. Agitation speed, pO2 and OD600 were determined and illustrated in Figure 1. The exponential phase started after 1.5 hours of cultivation. The cell growth caused a decrease in pO2. After 2 hours of cultivation the agitation speed increased up to 629 rmp (5.9 hours) to hold the minimal pO2 level of 50 %. Then, after 4 hours there was a break in cell growth due to induction of protein expression. The maximal OD600 of 2.78 was reached after 5 hours. In comparison to E. coli KRX (OD600,max =4.86 after 8.5 hours) and to E. coli KRX with BBa_K863000 (OD600,max =3.53 after 10 hours, time shift due to long lag phase) the OD600 max is lower. In the following hours, the OD600 and the agitation speed decreased and the pO2 increased, which indicates the death phase of the cells. This is caused by the cell toxicity of ECOL (reference: [http://www.dbu.de/OPAC/ab/DBU-Abschlussbericht-AZ-13191.pdf DBU final report]). Hence, cells were harvested after 12 hours.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 4444
Illegal BamHI site found at 1214
Illegal BamHI site found at 3313 - 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal AgeI site found at 1049
Illegal AgeI site found at 3148 - 1000INCOMPATIBLE WITH RFC[1000]Illegal SapI site found at 1031
Illegal SapI site found at 3130
