Difference between revisions of "Part:BBa K3895003"
(→Modeling) |
(→Modeling) |
||
| Line 10: | Line 10: | ||
|[[File:T--SZ SHD--Z50.1.gif|200px|thumb|center|'''Figure 1.''' Swiss Model showing serine residues of KerAVDZ50.]] | |[[File:T--SZ SHD--Z50.1.gif|200px|thumb|center|'''Figure 1.''' Swiss Model showing serine residues of KerAVDZ50.]] | ||
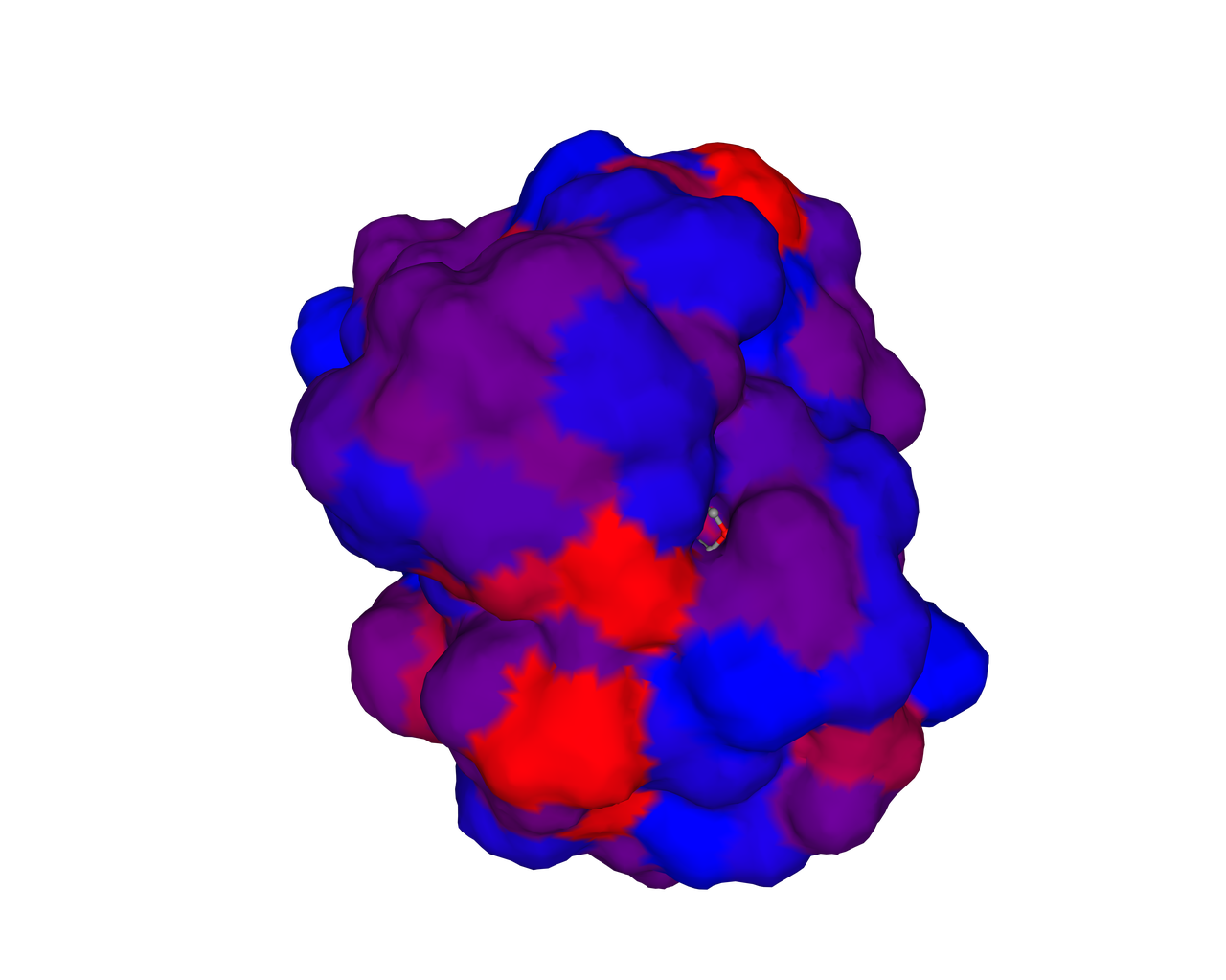
|[[File:T--SZ SHD--Z50.2.png|200px|thumb|center|''' Figure 2.''' Swiss Model showing hydrophobisity of KerAVDZ50.]] | |[[File:T--SZ SHD--Z50.2.png|200px|thumb|center|''' Figure 2.''' Swiss Model showing hydrophobisity of KerAVDZ50.]] | ||
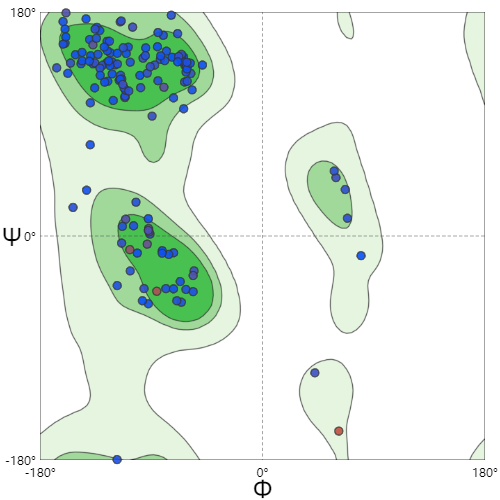
| + | |[[File:T--SZ SHD--Z50R.jpg|200px|thumb|center|''' Figure 3.''' Ramachandran Plot of KerAVDZ50.Z50 Ramachandran Favoured is 95.14%]] | ||
|} | |} | ||
Revision as of 15:14, 10 October 2021
Keratinase kerAvDZ50
KerAVDZ50 is an extracellular serine thiol alkaline protease from Actinomadura viridilutea strain, which can be isolated from Algerian fishing port [1]. As a thermo-alkaline keratinases, it can function at a pH range of 7–12 and a temperature range of 35–80 °C.
Modeling
Sequence and Features
Assembly Compatibility:
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 577
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal AgeI site found at 382
Illegal AgeI site found at 1120 - 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI.rc site found at 322
Reference
Biochemical and molecular characterization of new keratinoytic protease from Actinomadura viridilutea DZ50. (n.d.). International Journal of Biological Macromolecules, 92, 299–315. https://doi.org/10.1016/j.ijbiomac.2016.07.009