Difference between revisions of "Part:BBa K3410004"
| Line 1: | Line 1: | ||
| − | |||
__NOTOC__ | __NOTOC__ | ||
<partinfo>BBa_K3410000 short</partinfo> | <partinfo>BBa_K3410000 short</partinfo> | ||
| Line 43: | Line 42: | ||
<partinfo>BBa_K3410002 parameters</partinfo> | <partinfo>BBa_K3410002 parameters</partinfo> | ||
<!-- --> | <!-- --> | ||
| − | |||
Revision as of 17:57, 27 October 2020
Flavin-based fluorescent protein (FbFP B2)
The progesterone nanobody has been grafted in silico by our iGEM team. Our grafting method was based on the publication of Wagner et al. [1] .It was grafted using the scaffold provided by the cAbBCII-10 Nanobody (3DWT; BBa_K3410001) (acceptor) [2] and the CDR 1-3 of a monoclonal antibody against progesterone (Donor). As CDR Donor we used P15G12C12G11 [3, 4]. As this nanobody is grafted in silico it is only a low affinity binder against its target hormone. Accordingly, it would be necessary to subject the nanobody to an affinity mutation for future use. A phage display would be suitable for this purpose, or more precisely a phagemid display, in which the sequence provided in the part would first have to be mutated [1].
<img class "figure image" scr" ">
">
Figure 4: Schematic representation of the in silico grafting process. To start grafting, an acceptor and a suitable CDR donor must be selected. The CDRs 1 to 3 are transferred from the donor (any antibody) to the acceptor (a nanobody suitable for grafting). The resulting initial graft is usually a weak binder. The grafted sequence must therefore be subjected to a suitable affinity maturation method, for example a phage display. After affinity maturation a new synthetic nanobody with high affinity is obtained. Abbreviations: CDR, complementarity-determining region; VH, variable domain of the heavy chain; VHH, single variable domain on a heavy chain antibody; VL, variable domain of the light chain. Created with BioRender.
</br>
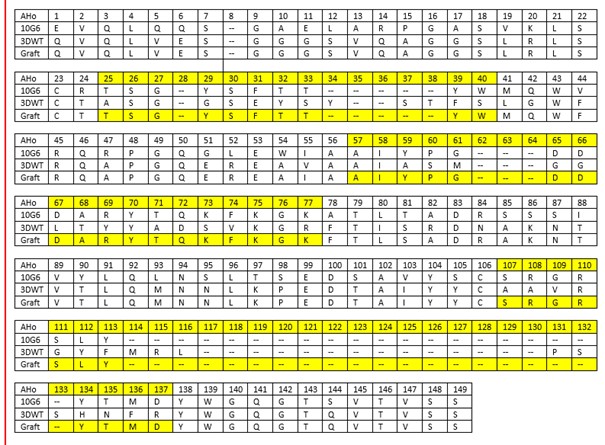
Table 1: Design of the estradiol nanobody. The amino acid residues (AHo numbering) of the donor antibody (10G6D6), the accepting antibody (3DWT) and the grafted estradiol nanobody are shown. The complementarity determining regions are highlighted in yellow.
References
[1] H. J. Wagner, S. Wehrle, E. Weiss, M. Cavallari, and W. Weber, “A Two-Step Approach for the Design and Generation of Nanobodies,” International journal of molecular sciences, vol. 19, no. 11, 2018, doi: 10.3390/ijms19113444.
[2] C. Vincke, R. Loris, S. Muyldermans, and K. Conrath, Structure of CabBCII-10 nanobody, 2008.
[3] C. Monnet et al., Crystal Structure of Fab-Estradiol Complexes, 2002.
[4] C. Monnet et al., “Highly specific anti-estradiol antibodies: structural characterisation and binding diversity,” Journal of molecular biology, vol. 315, no. 4, pp. 699–712, 2002, doi: 10.1006/jmbi.2001.5284.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
